Understanding Risk Assessment in Pharmaceutical Quality Management
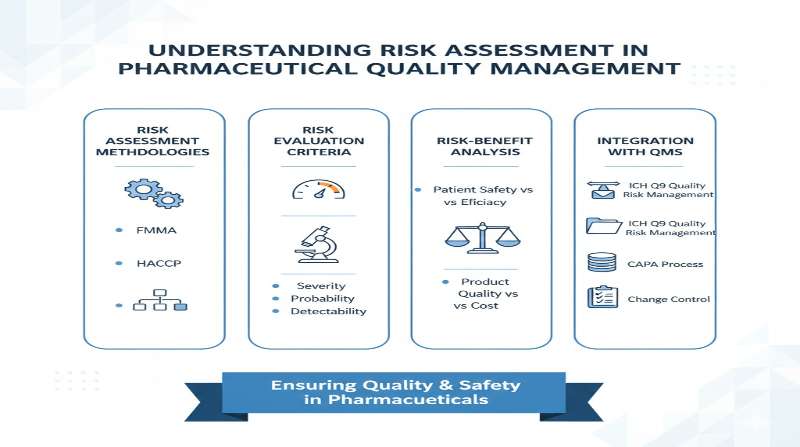
What is Risk Assessment?
Since 2002, when the FDA launched its landmark “Pharmaceutical CGMPs for the 21st Century” initiative, and formally implementing it through a comprehensive report in 2004, the agency has adopted what is known as a risk-based approach to pharmaceutical oversight and quality management. This strategic shift represents a fundamental transformation in how pharmaceutical quality is regulated and maintained. Under this risk-based approach, pharmaceutical companies are required to conduct systematic risk assessments as an integral part of their quality management systems.
The risk-based approach was further strengthened with the publication of the International Council for Harmonisation (ICH) Q9 guideline on Quality Risk Management in 2006, which was subsequently revised and updated as ICH Q9(R1) in January 2023 (implemented in May 2023 in the United States and July 2023 in Europe). This revised guideline addresses key challenges identified in the original guidance, including high levels of subjectivity in risk assessments, lack of clarity on risk-based decision-making, insufficient understanding of formality requirements in quality risk management work, and product availability risks due to manufacturing quality issues.
But what exactly does risk assessment entail in the pharmaceutical context?
A Practical Example: Understanding Risk in Food Preparation
To illustrate the concept clearly, let us consider a straightforward example from a hamburger shop. Imagine a manufacturing process where hamburgers must be cooked according to specific parameters.
The manufacturing instruction specifies: “Heat at 180°C for 3 minutes.”
Identifying the Hazards and Associated Risks
The critical question we must ask is: what are the potential consequences if these parameters are not met? If the temperature fails to reach 180°C, or if the heating duration falls short of 3 minutes, the heat may not penetrate to the center of the hamburger patty, resulting in inadequate bacterial elimination. This creates a significant food safety hazard that could lead to foodborne illness outbreaks among consumers.
Analyzing Failure Modes
To conduct a comprehensive risk assessment, we must systematically examine all potential failure modes—the various ways in which our process could deviate from the intended outcome. For the temperature requirement of 180°C, potential failure modes include:
- Burner malfunction (visible components may appear normal while internal blockages or defects compromise performance)
- Temperature sensor calibration drift or complete failure
- Human error in reading or interpreting temperature gauge displays
- Environmental factors affecting heat distribution
Similarly, for the time requirement of 3 minutes, we must consider:
- Timer malfunction or inaccurate calibration
- Human error in reading time displays or starting/stopping the timer
- Operator distraction or interruption during the timing process
The Three Components of Risk
According to ICH Q9(R1), risk is formally defined as the combination of the probability of occurrence of harm and the severity of that harm. In our hamburger example, we are evaluating both:
- Probability: How likely is it that the temperature or time requirement will not be met?
- Severity: What are the potential health consequences if undercooked hamburgers reach consumers?
This systematic approach to identifying potential failure events through the lens of equipment malfunction and human error must be applied to every process in pharmaceutical manufacturing. The pharmaceutical industry must conduct similar assessments across all critical manufacturing steps, considering how process parameters affect product quality attributes, and ultimately, patient safety.
Developing Risk Control Strategies
Once risks have been identified and assessed, we can develop appropriate control strategies to mitigate them. This is where the value of risk assessment becomes evident—understanding the risks enables us to implement targeted, effective countermeasures.
Implementing Control Measures
For the hamburger cooking example, appropriate risk control measures might include:
Equipment Controls:
- Establish a preventive maintenance program with daily visual and functional inspection of burners
- Implement routine calibration verification of temperature sensors and timers
- Install redundant monitoring systems (dual temperature sensors and timers operating independently)
Process Controls:
- Define critical process parameters and their acceptable ranges
- Establish sampling and testing frequencies to verify that parameters are consistently met
- Implement statistical process control to detect trends before they result in out-of-specification conditions
Human Factor Controls:
- When necessary for high-risk operations, require dual operator verification of critical readings
- Implement automated alerts when parameters deviate from specified ranges
- Provide comprehensive training on the importance of these parameters to product safety
- Design displays and interfaces to minimize reading errors (e.g., digital displays, color-coded alarm zones)
Risk-Based Decision-Making
The ICH Q9(R1) revision emphasizes that the level of effort, formality, and documentation applied during quality risk management should be commensurate with the level of risk. This concept exists on a continuum from less formal approaches for lower-risk situations to highly structured, documented processes for higher-risk scenarios. The guideline clarifies that resource constraints should never be used to justify inadequate formality in the risk management process when the level of risk warrants more rigorous approaches.
Risk-based decision-making can range along a spectrum:
- Highly structured approaches: Involve formal decision criteria, quantitative analysis where possible, detailed documentation, and multi-disciplinary review
- Less structured approaches: May rely more on expert judgment, qualitative assessment, and streamlined documentation
The appropriate level of structure should be determined by factors such as uncertainty, importance, and complexity of the situation, not by resource availability.
Managing Subjectivity in Risk Assessment
One of the key improvements in ICH Q9(R1) is the emphasis on managing and minimizing subjectivity in risk assessments. The guideline acknowledges that some degree of subjectivity is inherent in risk management but provides strategies to enhance objectivity:
- Base risk scores and ratings on appropriate use of evidence, scientific principles, and accumulated knowledge rather than solely on intuition
- Make assumptions and uncertainties explicit and transparent in risk documentation
- Use cross-functional teams to provide diverse perspectives and challenge assumptions
- Validate risk assessment approaches through comparison with actual outcomes and historical data
- Apply appropriate risk management tools selected based on the specific situation rather than using a one-size-fits-all approach
Integration with Pharmaceutical Quality Systems
Risk assessment and risk management are not standalone activities but are integrated throughout the pharmaceutical product lifecycle:
| Lifecycle Stage | Risk Management Applications |
| Development | Identifying critical quality attributes; designing robust manufacturing processes; establishing control strategies |
| Technology Transfer | Assessing risks associated with process scale-up and site changes; validating process capabilities |
| Routine Manufacturing | Monitoring process performance; investigating deviations; implementing corrective and preventive actions |
| Continuous Improvement | Evaluating proposed changes; implementing process improvements; managing supply chain risks |
| Product Discontinuation | Managing risks associated with product transitions; ensuring continued supply during changeovers |
Furthermore, ICH Q9(R1) introduces enhanced guidance on quality risk management as part of supply chain control, recognizing the increasing complexity and globalization of pharmaceutical supply chains. This includes systematic assessment of supplier capabilities, monitoring of supplier performance, and proactive management of supply continuity risks.
The Broader Impact: Addressing Product Availability
A significant addition in ICH Q9(R1) is explicit guidance on using quality risk management to address product availability risks arising from quality and manufacturing issues. Drug shortages have emerged as a critical public health concern, and the revised guideline emphasizes that risk management should consider not only product quality but also the potential for manufacturing disruptions to affect patient access to medicines. This requires:
- Proactive identification of vulnerable points in the manufacturing process or supply chain
- Development of contingency plans for critical process steps
- Consideration of manufacturing site capacity and redundancy
- Assessment of raw material sourcing risks
- Evaluation of the impact that quality issues at one site could have on global supply
Conclusion
Risk assessment in pharmaceutical manufacturing follows the same fundamental principles illustrated by our hamburger cooking example, but with far greater complexity and stringency appropriate to the critical nature of pharmaceutical products. By systematically identifying potential failure modes, evaluating their likelihood and impact, and implementing appropriate controls, pharmaceutical companies fulfill their responsibility to patients and regulatory authorities.
The evolution from ICH Q9 to ICH Q9(R1) reflects the pharmaceutical industry’s growing maturity in applying risk management principles. The revised guideline’s emphasis on objectivity, appropriate formality, clear decision-making frameworks, and consideration of product availability demonstrates that risk management continues to evolve as a cornerstone of modern pharmaceutical quality systems.
When pharmaceutical companies thoroughly understand and properly apply risk assessment principles—making risk management an integral part of their quality culture rather than a compliance checkbox—they can ensure both the quality of their products and their availability to the patients who depend on them. This systematic, science-based approach to managing risk represents the foundation of modern pharmaceutical quality assurance and continues to be refined as our understanding and regulatory expectations advance.
Related Articles
- The Necessity of Risk-Based Approach
- Quality Risk Management: Understanding ICH Q9 and the Revised GMP Regulations
- Quality Risk Management in the Pharmaceutical Industry
- Tips for Risk Management
- What is Risk Analysis?
Related FDA QMSR Templates
Streamline your FDA QMSR compliance with our professionally crafted templates:



Comment