What is Risk Analysis?
In the risk assessment process explained previously, the first activity to be performed is “risk analysis.”
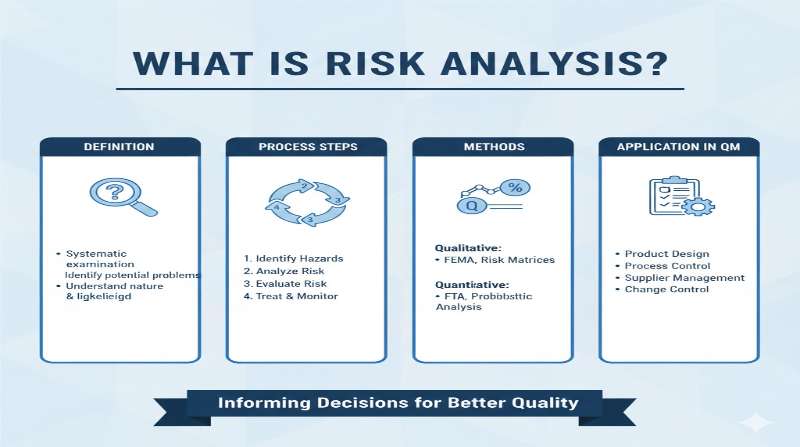
Definition of Risk Analysis
The definition of risk analysis is described in the ICH Q9(R1) “Quality Risk Management” guideline (effective July 2023) as follows:
“The estimation of the risk associated with the identified hazards.”
Risk analysis is the qualitative or quantitative process of linking the probability of occurrence of harm and the severity of that harm. In some risk management tools, the detectability of harm is also factored into the risk estimation.
What is a Hazard?
A hazard refers to a potential source of harm. The same definition is provided in ISO 14971:2019 (Application of Risk Management to Medical Devices), making it an important concept common to the pharmaceutical and medical device fields.
Practical Example: Risk Analysis of a PC Projector
Let us explain the process from hazard to harm using a PC projector as an example.
Hazards Associated with a PC Projector
A PC projector has the following hazards:
- Light (high-intensity light during projection)
- Heat (device heating during projection)
- Hot air (high-temperature air expelled from cooling fan)
- Electrical power (electrical energy)
- Electromagnetic waves (electromagnetic fields during operation)
- Gravity (risk of falling when suspended from ceiling, etc.)
Sequence from Hazard to Harm
Taking “heat” as an example, risk can be analyzed through the following sequence:
- Hazard: Heat (device heating)
- Hazardous situation: The device surface becomes hot due to prolonged use or poor ventilation
- Exposure to hazard: A user comes into contact with the hot PC projector
- Harm: Burns (physical injury to the body)
This systematic inference and analysis of the causal chain from “hazard” → “hazardous situation” → “exposure” → “harm” is called “risk analysis.”
Importance of Risk Analysis
Risk analysis is a core process in the three steps of risk assessment (risk identification, risk analysis, and risk evaluation). Conducting proper risk analysis enables:
- Early detection of potential harms
- Design of effective risk control measures
- Enhancement of patient safety
- Compliance with regulatory requirements
- Evidence-based decision making
Risk Analysis Methods
ICH Q9(R1) presents various tools that can be used for risk analysis. Representative methods include:
| Method | Characteristics | Application |
| FMEA (Failure Mode and Effects Analysis) | Evaluates potential failure modes and their effects | Manufacturing processes, product design |
| FTA (Fault Tree Analysis) | Deductively analyzes causes of undesirable events | Failure analysis of complex systems |
| HAZOP (Hazard and Operability Study) | Systematically examines process deviations | Chemical processes, manufacturing operations |
| HACCP (Hazard Analysis and Critical Control Points) | Identifies and controls critical control points | Quality control in manufacturing processes |
It is important to select appropriate methods according to the situation or use them in combination.
Latest Regulatory Trends
ICH Q9 was revised as the R1 version in 2023, with the following enhancements:
- Clarification of responses to product availability risks
- Emphasis on the importance of risk-based decision making
- Management and minimization of subjectivity in risk assessments
- Consideration of product availability risks due to manufacturing quality issues
These revisions require more comprehensive and scientifically grounded risk management implementation.
Conclusion
Risk analysis is the process of systematically estimating the probability of occurrence and severity of harm arising from identified hazards. By conducting appropriate risk analysis, it becomes possible to ensure patient safety and provide high-quality pharmaceuticals and medical devices.
Related Articles
- The Necessity of Risk-Based Approach
- Quality Risk Management: Understanding ICH Q9 and the Revised GMP Regulations
- Quality Risk Management in the Pharmaceutical Industry
- Tips for Risk Management
- Understanding Risk Assessment in Pharmaceutical Quality Management
Related FDA QMSR Templates
Streamline your FDA QMSR compliance with our professionally crafted templates:



Comment