Fundamental Principles of Quality Assurance in System Development
In system development, ensuring quality stands as one of the most critical challenges. This importance becomes particularly acute in systems related to pharmaceutical manufacturing and medical device production, where direct impacts on human health and life are at stake. The V-model development methodology emerged from this backdrop as a structured approach to managing quality throughout the system lifecycle.
Understanding the V-Model
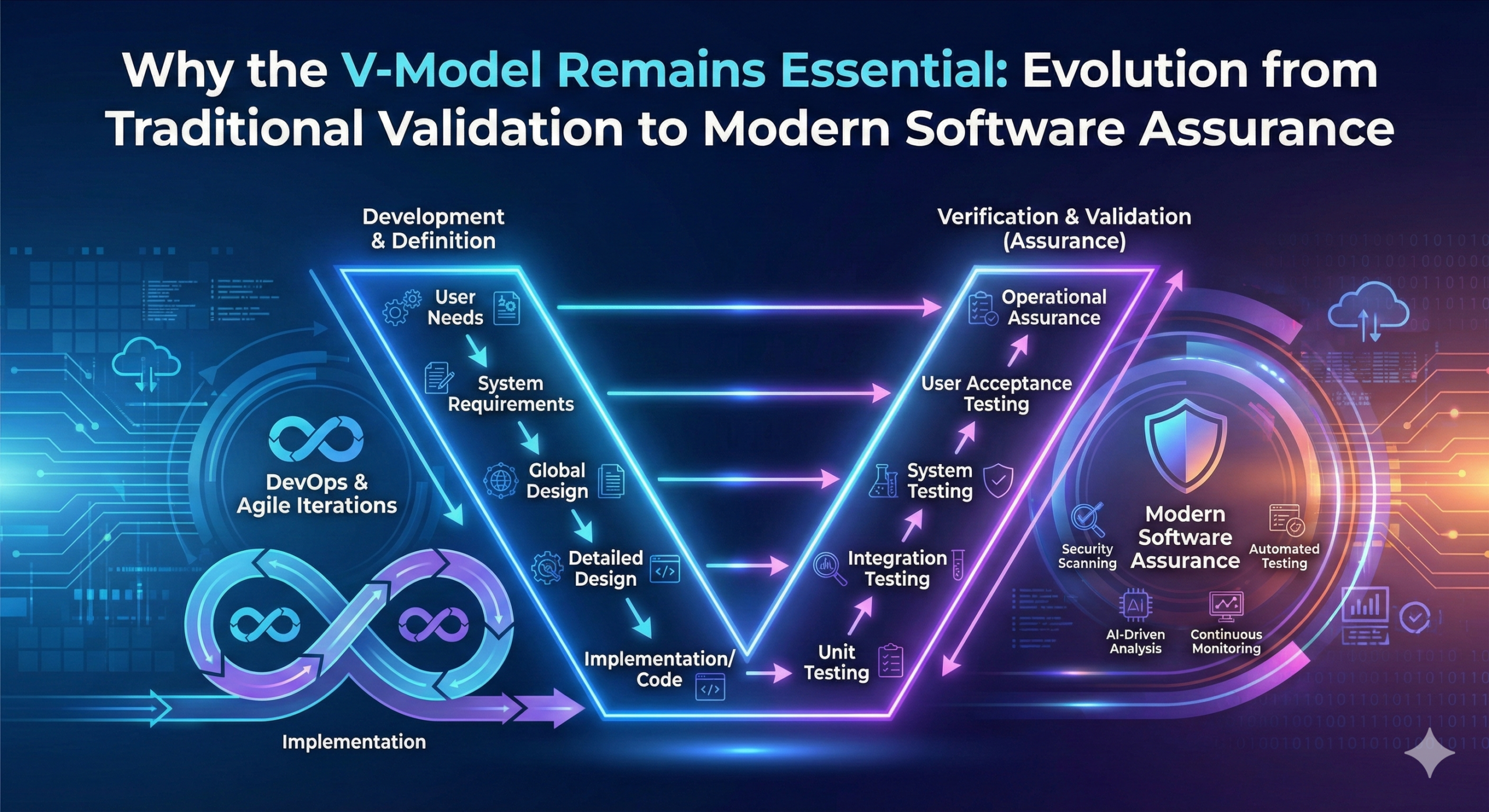
The V-model, named for its characteristic “V” shape, represents the system development process visually. The left descending line represents the specification (design) phases, while the right ascending line represents the verification (testing) phases. The model’s defining characteristic is its fundamental principle: “Each development phase deliverable (specification) has a clearly defined corresponding verification activity.”
Left Side: Specification Phases
On the left side of the V-model, the process begins with system requirements and progressively elaborates into detailed specifications. The typical progression includes:
User Requirements Specification (URS): Defines what the system must accomplish
Functional Specification (FS): Defines what the system will do
Design Specification (DS): Defines how the system will be implemented
Module Specification: Detailed design of individual components
The specification documents created at each stage become inputs for the next stage, transforming into increasingly concrete and technical content as development progresses.
Right Side: Verification Phases
The right side of the V-model conducts verification activities corresponding to each specification created on the left. This ensures that all specifications are properly implemented:
Module Testing: Verifies that individual components operate according to design
Integration Testing: Verifies that component interactions follow design specifications
System Testing: Verifies that the complete system meets functional specifications
Acceptance Testing: Verifies that user requirements are satisfied
The Critical Importance of This Principle
The principle that “each specification has a clearly defined corresponding verification” may appear self-evident at first glance. However, in actual projects, many problems arise precisely when this correspondence becomes unclear.
Prevention of Verification Gaps
By implementing corresponding verification on the right side for every specification defined on the left, verification gaps can be systematically prevented. For instance, if no test cases exist for a particular functional requirement, there is no mechanism to confirm whether that function is correctly implemented. The V-model provides a structural framework to prevent such omissions.
Ensuring Traceability
By clearly linking each specification with its corresponding verification activity, the entire chain from requirements to final test results becomes traceable. This traceability proves especially critical in regulated industries. When issues arise, it enables clear identification of which requirement is involved and which test should have detected the problem.
GAMP 5 and the Evolution to Risk-Based Approaches
Good Automated Manufacturing Practice (GAMP) serves as a guideline for computerized system validation in the pharmaceutical industry. The evolution from GAMP 5 First Edition (2008) to the Second Edition (July 2022) reflects significant changes in both technology and regulatory thinking, while maintaining the V-model as a foundational framework.
GAMP 5 Second Edition: Modernization and Critical Thinking
The GAMP 5 Second Edition, published in July 2022 after 14 years since the first edition, introduces substantial updates to reflect the evolving technological and regulatory landscape. The guide maintains the principles and risk-based framework of the first edition while modernizing its application to better align with current software engineering practices and regulatory expectations.
Key updates in the Second Edition include:
Emphasis on Critical Thinking: The Second Edition highlights the use of critical thinking by knowledgeable subject matter experts (SMEs) to define appropriate approaches, prioritizing patient safety and product quality over mere compliance.
Support for Modern Development Methodologies: The guide explicitly acknowledges and supports agile, iterative, and DevOps methods, clarifying that the GAMP specification and verification approach is not inherently linear but fully supports incremental methods.
Computer Software Assurance (CSA): One of the most significant changes is the shift in emphasis from traditional Computerized System Validation (CSV) to Computer Software Assurance (CSA), driven by the FDA’s Case for Quality initiative.
New Technologies: The Second Edition includes new appendices addressing artificial intelligence and machine learning (AI/ML), blockchain technologies, cloud computing, and open-source software.
Service Provider Focus: The guide recognizes the increased importance of service providers, including cloud service providers, and encourages regulated companies to maximize supplier involvement to leverage their knowledge, experience, and documentation.
Software Categorization: A Refined Approach
GAMP categorizes systems to apply appropriate levels of validation rigor based on complexity and risk. The current GAMP 5 maintains four categories (Category 2 for firmware was discontinued as modern firmware can fit into other categories depending on its nature):
Category 1: Infrastructure Software: Generic, widely used infrastructure software including operating systems, database servers, programming languages, middleware, and office suite applications. These platforms are not designed specifically for GxP tasks but provide foundational support. The Second Edition significantly updated the definition to focus on software, systems, and tools supporting computerized system lifecycle activities and IT infrastructure processes, aligning with the FDA CSA approach.
Category 3: Non-Configured Products: Software without configurable functions, marketed freely or integrated into hardware, such as tools for statistical calculation or data acquisition software without configuration capability.
Category 4: Configured Products: Configurable commercial off-the-shelf software where specific parameters are set to adapt to user needs without modifying original functionalities.
Category 5: Custom Applications: Fully custom, bespoke software developed in-house or by contractors, representing the highest complexity and requiring rigorous control throughout all development phases.
The following table summarizes the validation approach for each category:
| Category | Type | Description | Validation Focus | Documentation Level |
|---|---|---|---|---|
| 1 | Infrastructure Software | Operating systems, databases, development tools | Installation verification, version documentation | Minimal – qualification rather than validation |
| 3 | Non-Configured Products | Standard COTS software used “as-is” | Functional testing of standard features, vendor assessment | Moderate – leverage vendor documentation |
| 4 | Configured Products | Configurable COTS software | Configuration testing, functional testing | Substantial – document configuration and testing |
| 5 | Custom Applications | Bespoke developed systems | Full lifecycle testing from requirements through deployment | Extensive – complete specification and verification |
Higher categories require progressively more detailed specification and verification activities. The Second Edition emphasizes that categories should be viewed as a continuum rather than rigid classifications, and that other factors such as system criticality, complexity, and novelty also drive risk-based approaches.
Achieving Efficient Validation Through Risk-Based Approaches
The category-based approach enables validation at appropriate levels based on system complexity and risk. Rather than applying the same level of rigor to all systems, this risk-based approach allows organizations to focus on what truly matters for patient safety, product quality, and data integrity. This provides several advantages:
Optimal Resource Allocation: Higher-risk systems receive more intensive scrutiny and resources.
Reduced Development Time: Lower-risk systems focus on essential activities, avoiding unnecessary work.
Cost Reduction: By eliminating excessive documentation and testing that does not add value, costs are reduced while maintaining quality.
Quality Maintenance: Appropriate levels of quality assurance are applied based on actual risk rather than prescriptive requirements.
The FDA Computer Software Assurance Paradigm
In September 2022, the FDA issued draft guidance on “Computer Software Assurance for Production and Quality System Software,” which was finalized in September 2025. This guidance describes a risk-based approach to establish confidence in automation used for production or quality systems, focusing on patient safety, product quality, and data integrity.
The Four-Step CSA Framework
Computer software assurance follows a four-step process that considers the risk of compromised safety or quality should the software fail to perform as intended:
1. Identify Intended Use: Clearly define how the software will be used within production or quality systems.
2. Determine Risk-Based Approach: Assess the potential impact on device safety, quality, and record integrity to determine appropriate assurance levels.
3. Determine Appropriate Assurance Activities: Select testing and verification activities commensurate with risk. For high process risk features, rigorous scripted testing may be appropriate, while not-high-process-risk features may utilize unscripted testing methods such as scenario testing, error-guessing, or exploratory testing.
4. Establish Appropriate Record: Document assurance activities including intended use, risk determination, what was tested, by whom, and the results, while leveraging digital records, system logs, and audit trails to reduce manual documentation burden.
Alignment with GAMP 5 Second Edition
The FDA CSA guidance aligns closely with GAMP 5 Second Edition, which was developed concurrently and incorporates the same critical thinking principles. Both emphasize a focus on clear thinking through planning, then creating documentation from a process perspective, rather than the traditional CSV approach of generating extensive paper-based testing records.
CSA represents a least-burdensome approach where validation burden is no more than necessary to address actual risk, supporting efficient use of resources while promoting product quality. This philosophy encourages:
- Leveraging vendor assessments and certifications
- Using digital records over manual documentation
- Applying critical thinking to determine truly necessary testing
- Focusing resources on patient safety and data integrity risks
Practical Application of the V-Model in Modern Contexts
While the V-model provides the structural framework, its application must adapt to contemporary software development practices and regulatory expectations. The following considerations are essential for effective implementation:
Early Planning and Critical Thinking
In the project’s initial phase, it is critical to apply critical thinking to determine which specification documents are necessary and what verification activities will be performed for each, documented in the Validation Plan (VP). Rather than reflexively following prescriptive checklists, teams should think through the plan, focusing on quality aspects where the most risk lies, and then create documentation from a process perspective.
Continuous Review and Quality Gates
Deliverables from each phase must be appropriately reviewed before proceeding to the next phase. This enables early problem detection and minimizes rework. The Second Edition emphasizes moving away from habitual creation of paper documentation as validation deliverables toward critical risk-based evaluation of evidential records created during concept, project, operation, and retirement phases.
Integration with Change Management
When specification changes occur during system development, corresponding verification activities must be updated accordingly. The V-model provides a framework for visualizing and managing such change impacts, ensuring traceability from requirements through implementation to testing.
Adaptation to Agile Methodologies
The GAMP 5 Second Edition explicitly supports agile, iterative approaches, clarifying that validation can and should be applied on a sprint-by-sprint basis in agile projects. Rather than creating separate Waterfall-style User Requirements Specifications, agile teams should use the sprint backlog as the single source of truth, with change logs and status changes serving as approvals with audit trails providing evidence.
The V-model principles remain valid in agile contexts:
- User stories and acceptance criteria replace traditional URS documents
- Sprint planning incorporates specification activities
- Definition of Done includes verification criteria
- Sprint reviews provide the verification activities
- Retrospectives ensure continuous improvement
Leveraging Supplier Involvement
Modern computerized systems increasingly rely on cloud service providers and external vendors. GAMP 5 Second Edition encourages establishing comprehensive service level agreements and conducting thorough supplier evaluations to ensure compliance throughout the system lifecycle. This approach:
- Reduces redundant testing by leveraging vendor testing and certifications
- Focuses internal efforts on configuration, integration, and business process validation
- Establishes clear responsibilities between suppliers and regulated companies
- Maintains appropriate oversight while avoiding duplication
Contemporary Regulatory Landscape
Global Harmonization Trends
The FDA’s Quality System Regulation is harmonizing with ISO 13485, further aligning U.S. requirements with global standards. This harmonization supports:
- Consistent approaches across jurisdictions
- Reduced compliance burden for global operations
- Clearer expectations for manufacturers
- Enhanced focus on quality management systems
Data Integrity as a Central Concern
Modern guidance emphasizes data integrity alongside patient safety and product quality as fundamental concerns. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) guide computerized system design and validation to ensure data reliability throughout the lifecycle.
Cybersecurity Considerations
The GAMP 5 Second Edition provides updated guidance on cybersecurity, reflecting the increased threat landscape and regulatory focus on protecting systems and data from malicious actors. Security considerations must be integrated throughout the V-model:
- Security requirements defined in specification phases
- Security testing incorporated into verification phases
- Continuous monitoring throughout operational phases
- Incident response and recovery procedures
Practical Examples: Applying Modern V-Model Principles
Example 1: Laboratory Information Management System (LIMS)
For a Category 4 configured LIMS implementation:
Left Side (Specification):
- User Requirements: Define laboratory workflows, data requirements, reporting needs
- Functional Specification: Document how LIMS will support each workflow, interface requirements
- Configuration Specification: Detail specific parameter settings, user roles, workflow configurations
Right Side (Verification):
- Configuration Testing: Verify each configuration parameter operates as specified
- Integration Testing: Test interfaces with instruments and other systems
- User Acceptance Testing: Verify laboratory workflows function as required
CSA Approach: Leverage vendor’s ISO 13485 certification and development testing, focus internal testing on configuration, integration, and workflows that directly impact data integrity and product quality decisions.
Example 2: Custom Manufacturing Execution System (MES)
For a Category 5 custom MES:
Left Side (Specification):
- Detailed requirements for batch management, recipe control, equipment integration
- Complete functional and design specifications
- Module-level specifications for each component
Right Side (Verification):
- Comprehensive unit testing of all modules
- Integration testing with equipment, ERP, and LIMS
- System testing of complete manufacturing scenarios
- Performance qualification in production environment
CSA Approach: Apply rigorous scripted testing for high-risk features directly affecting batch integrity and product quality, while using unscripted testing for administrative functions and reporting that do not directly impact product.
Conclusion: The Enduring Relevance of the V-Model
The V-model is founded on a seemingly simple yet profoundly important principle: “Each specification has a clearly defined corresponding verification.” Application of this principle enables systematic and efficient quality assurance throughout system development. Particularly when utilizing frameworks such as GAMP 5 Second Edition and FDA Computer Software Assurance guidance, risk-based approaches enable efficient validation while maintaining focus on what truly matters—patient safety, product quality, and data integrity.
The V-model has evolved from the traditional linear waterfall approach to embrace modern methodologies including agile, DevOps, and cloud-based architectures, while maintaining its fundamental principle of specification-verification correspondence. The transition from Computer System Validation (CSV) to Computer Software Assurance (CSA) represents not an abandonment of the V-model but rather its modernization—shifting focus from exhaustive documentation to critical thinking about risk, quality, and patient safety.
In fields demanding high quality such as pharmaceutical manufacturing and medical device development, the V-model represents far more than a mere development methodology. It constitutes an essential framework for protecting patient safety, satisfying regulatory requirements, and achieving efficient development. By encouraging the application of modern IT practices, robust quality risk management approaches, and excellence in software engineering, contemporary guidance seeks to meet and exceed minimum compliance expectations for the benefit of patients and public health.
As all stakeholders involved in system development understand and appropriately apply these evolved principles, they enable construction of systems that are safer, more reliable, and better suited to protecting the patients who ultimately depend on them. The V-model, when combined with critical thinking, risk-based approaches, and modern development practices, remains not only relevant but essential in today’s rapidly evolving technological and regulatory landscape.

Comment