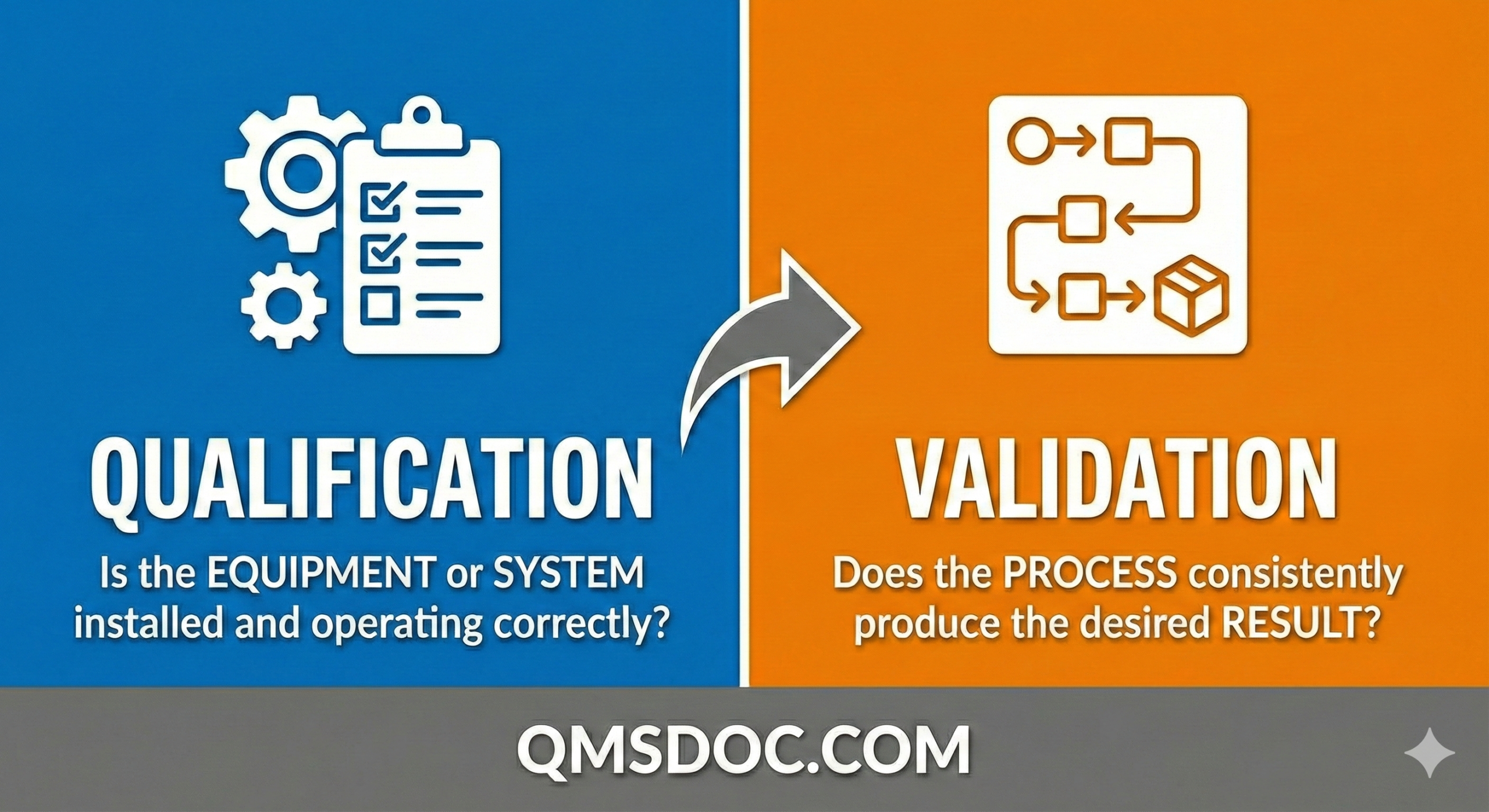
The Difference Between Qualification and Validation
Regulatory Context and Recent Updates
The revised GMP Ministerial Ordinance of Japan, which came into effect on August 1, 2021, introduced significant amendments to the “Validation Standards.” This revision was designed to harmonize Japanese GMP requirements with the 2015 edition of PIC/S GMP Annex 15, titled “Qualification and Validation.”
It is important to note that PIC/S (Pharmaceutical Inspection Co-operation Scheme) has continued to update its guidance. The most recent revision of Annex 15 was published in 2023, and the current version of Annex 11 on Computerised Systems was revised in 2022. These ongoing updates reflect the evolution of pharmaceutical manufacturing technologies and risk-based approaches to quality assurance. Additionally, the ICH Q12 guideline on “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management,” which was finalized in 2019, has further shaped modern validation approaches by emphasizing product lifecycle management and the establishment of control strategies.
Hardware and Software: The Foundation of GMP
The concepts of “Hardware” and “Software” are indispensable elements in understanding GMP principles. Both GMP Hardware and GMP Software work together to achieve the three fundamental principles of Good Manufacturing Practice.
GMP Hardware
Hardware refers to manufacturing facilities, equipment, and instruments. GMP Hardware must fulfill the following requirements to support the three GMP principles:
Principle 1 – Error Prevention: Manufacturing facilities must have equipment and environments designed to prevent human errors and mix-ups. This includes proper layout design, segregation of operations, and equipment that minimizes the potential for mistakes.
Principle 2 – Contamination Prevention: Facilities must maintain hygienic equipment and environments to prevent contamination. This encompasses cleanroom classification, material compatibility, ease of cleaning, and appropriate environmental control systems.
Principle 3 – Consistent High Quality: Manufacturing facilities must have equipment and environments capable of maintaining consistently high quality. This requires robust design, appropriate monitoring systems, and equipment capable of operating within validated parameters over extended periods.
GMP Software
GMP Software represents the procedural and documentary aspects of pharmaceutical manufacturing:
- Establishing rules and documenting them systematically (Standard Operating Procedures, specifications, protocols)
- Implementing procedures according to established rules and creating comprehensive records (batch records, logbooks, deviation reports)
- Conducting periodic reviews and implementing continuous improvements (Change control, CAPA systems, management review)
The synergy between GMP Hardware and GMP Software is essential for achieving and maintaining pharmaceutical quality standards.
Computerised Systems in Modern GMP
GMP Hardware is primarily composed of physical equipment and facilities. However, in contemporary pharmaceutical manufacturing, most structural equipment is controlled by software programs embedded within the hardware. These may include Programmable Logic Controllers (PLCs), firmware, or other software systems (collectively referred to as “software” in this context).
A computerised system is defined as GMP Hardware that is controlled or influenced by software. The International Society for Pharmaceutical Engineering (ISPE) defines a computerised system as “a system including the input of data, electronic processing, and the output of information to be used either for reporting or automatic control.”
Critical Principles for Computerised Systems
The fundamental principle governing computerised systems is that the quality of manufactured (or analyzed) pharmaceutical products and the quality assurance system must not be compromised when manual operations are replaced by software-controlled processes. Put simply, automation should maintain or improve product quality—it must never diminish it.
To ensure this principle is upheld, Computerised System Validation (CSV) must be performed. The goal of CSV is to provide documented evidence that the software-controlled system produces products of equivalent or superior quality compared to manual processes, while maintaining the same level of quality assurance.
Moreover, computerisation and automation must not introduce new risks or increase existing risks to product quality, patient safety, or data integrity. The risk-based approach outlined in ICH Q9 (Quality Risk Management) should be applied throughout the lifecycle of computerised systems.
Data Integrity Considerations
In recent years, regulatory authorities worldwide have placed increased emphasis on data integrity within computerised systems. The MHRA (Medicines and Healthcare products Regulatory Agency) GMP Data Integrity Guidance, the FDA’s Guidance on Data Integrity and Compliance, and PIC/S PI 041-1 all stress the importance of ALCOA+ principles:
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
- Plus: Complete, Consistent, Enduring, and Available
These principles must be embedded in the design, implementation, and operation of all computerised systems used in GMP environments.
Qualification: Validation of GMP Hardware
Regardless of whether equipment is computerised, all GMP Hardware must undergo Qualification before use. Qualification is the systematic approach to demonstrating that equipment, facilities, and utilities are suitable for their intended purpose and consistently perform according to their specifications.
Qualification consists of four distinct stages:
Design Qualification (DQ): The documented verification that the proposed design of facilities, systems, and equipment is suitable for the intended purpose. DQ ensures that user requirements are properly translated into design specifications.
Installation Qualification (IQ): The documented verification that the facilities, systems, and equipment, as installed or modified, comply with the approved design and the manufacturer’s recommendations. IQ confirms that all components are correctly installed according to specifications.
Operational Qualification (OQ): The documented verification that the facilities, systems, and equipment, as installed or modified, perform as intended throughout the anticipated operating ranges. OQ demonstrates that the system operates correctly under various conditions.
Performance Qualification (PQ): The documented verification that the facilities, systems, and equipment, as connected together, can perform effectively and reproducibly based on the approved process method and product specification. PQ confirms that the system consistently produces acceptable results under routine operating conditions.
Qualification is typically performed once when equipment is initially installed, though requalification may be required after significant modifications, relocations, or as part of a periodic review program.
The Relationship Between CSV and Qualification
CSV cannot be performed in isolation because the software controls physical hardware. Therefore, CSV must be conducted as an integral part of equipment qualification. In practice, the CSV documentation often appears as one or more dedicated chapters or sections within the qualification report. For instance:
- The IQ phase includes verification of software installation and configuration
- The OQ phase includes testing of software functions and control logic
- The PQ phase includes verification that the software-controlled system performs correctly in actual production scenarios
This integrated approach ensures that both hardware and software components are validated together as a complete system.
Process Validation: The Complete Picture
Once GMP Hardware has been qualified, it is used in conjunction with GMP Software to perform Process Validation. Process validation provides documented evidence that a specific process consistently produces a product meeting predetermined specifications and quality attributes.
Process validation must be performed for each individual product (each formulation, strength, and dosage form). According to current regulatory thinking, process validation follows a lifecycle approach consisting of three stages:
Stage 1 – Process Design: The commercial manufacturing process is defined during this stage based on knowledge acquired through development and scale-up activities. This aligns with ICH Q8 (Pharmaceutical Development) principles and the Quality by Design (QbD) approach.
Stage 2 – Process Qualification: During this stage, the process design is evaluated to determine if the process is capable of reproducible commercial manufacturing. This typically includes the traditional concept of prospective validation runs.
Stage 3 – Continued Process Verification: Ongoing assurance is gained during routine production that the process remains in a state of control. This represents the modern concept of continuous process verification and is aligned with ICH Q10 (Pharmaceutical Quality System).
The Comprehensive Relationship
It is crucial to understand that:
- Process Validation encompasses both GMP Software and GMP Hardware
- Qualification is the specific form of validation applied to GMP Hardware
- CSV is integrated within Qualification for computerised systems
This relationship can be visualized as follows:
| Validation Scope | GMP Hardware | GMP Software | Timing | Scope |
| Qualification | Primary focus | Supporting documentation | Once per equipment (plus requalification as needed) | Equipment/system-specific |
| CSV | Integrated component | Integrated component | Part of Qualification | Software-controlled systems |
| Process Validation | Utilized (qualified) | Primary focus | Per product/process | Product/process-specific |
Addressing Industry Confusion
The interrelationships among CSV, Qualification, and Process Validation are rarely explained comprehensively in seminars, training programs, or published literature. This lack of clarity has been a significant source of confusion within the pharmaceutical industry.
Many organizations struggle with questions such as:
- Should CSV be performed separately from equipment qualification?
- When should process validation begin relative to equipment qualification?
- How should validation protocols and reports be structured?
- What is the relationship between validation and product lifecycle management?
Clarifying the Confusion
The key to understanding lies in recognizing the hierarchical and integrated nature of these activities:
- Foundation: Equipment Qualification (including CSV for computerised systems) ensures that the manufacturing tools are fit for purpose
- Application: Process Validation uses qualified equipment and documented procedures to demonstrate that a specific process consistently produces quality products
- Lifecycle: Both Qualification and Process Validation are part of the broader pharmaceutical quality system, requiring periodic review, revalidation when appropriate, and continuous verification
Modern Regulatory Expectations
Contemporary regulatory guidance emphasizes several key concepts:
Risk-Based Approach: Validation efforts should be proportionate to the risk to product quality and patient safety. ICH Q9 provides a framework for quality risk management that should be applied throughout qualification and validation activities.
Lifecycle Management: Equipment and processes require ongoing management throughout their lifecycle, not just initial validation. ICH Q10 and Q12 provide frameworks for maintaining validated status through change control, monitoring, and periodic review.
Data Integrity: As previously mentioned, ensuring data integrity is paramount in computerised systems. Validation must include provisions for maintaining ALCOA+ principles throughout the system lifecycle.
Knowledge Management: ICH Q10 emphasizes the importance of capturing, sharing, and using knowledge gained during development, validation, and commercial manufacturing to continuously improve processes.
Practical Implementation Considerations
For pharmaceutical companies implementing these concepts:
Develop Clear Validation Master Plans (VMP): The VMP should clearly define the organization’s approach to qualification and validation, including the integration of CSV within equipment qualification and the relationship to process validation.
Establish Integrated Documentation Systems: Qualification and validation documentation should be structured to clearly show the relationships among DQ, IQ, OQ, PQ, CSV, and process validation.
Implement Risk-Based Strategies: Not all equipment or processes carry the same level of risk. Validation rigor should be proportionate to the impact on product quality and patient safety.
Maintain Validated Status: Establish robust change control, periodic review, and continuous process verification programs to ensure that validated status is maintained throughout the product lifecycle.
Foster Cross-Functional Collaboration: Effective validation requires collaboration among quality assurance, engineering, production, and IT departments to ensure all aspects of GMP Hardware and Software are properly addressed.
Conclusion
Understanding the distinctions and relationships among Qualification, CSV, and Process Validation is essential for maintaining GMP compliance in modern pharmaceutical manufacturing. Qualification (including CSV as an integrated component) validates GMP Hardware, while Process Validation validates the complete manufacturing process using qualified equipment and documented procedures.
As regulatory expectations continue to evolve toward risk-based, lifecycle approaches, pharmaceutical manufacturers must maintain clarity on these fundamental concepts while adapting their validation strategies to meet modern standards. The integration of ICH guidelines (Q8, Q9, Q10, Q12) with PIC/S GMP Annexes provides a comprehensive framework for achieving and maintaining product quality through effective qualification and validation programs.
Organizations that develop clear validation strategies, maintain integrated documentation systems, and foster cross-functional collaboration will be well-positioned to meet current and future regulatory expectations while ensuring the consistent production of high-quality pharmaceutical products.
Note: This article provides educational information on qualification and validation principles in pharmaceutical manufacturing. Organizations should consult current regulatory guidance documents and seek expert advice when developing their specific validation strategies.

Comment