The Difference Between Validation and Verification
Introduction
The author frequently receives questions such as “What is the difference between validation and verification?” The FDA clearly distinguishes between “verification” and “validation” as separate and distinct terms. On the other hand, many industries use the terms “verification” and “validation” interchangeably, or refer to them as a unified concept such as “V&V,” suggesting that no strict distinction exists. This article explains the differences between validation and verification as defined by the FDA and the international standard ISO 13485.
Definitions in FDA and ISO 13485
Definition of Verification
The FDA defines verification in 21 CFR 820.3(aa) as follows:
“Confirmation by examination and provision of objective evidence that specified requirements have been fulfilled.”
ISO 13485:2016 also treats verification as a similar concept.
Specifically, verification means:
- Confirming that a product has been manufactured according to design specifications
- Confirming that a process has been controlled as specified
- Confirming that a product conforming to specifications has been manufactured
Verification is an activity performed on products after manufacturing, confirming that design outputs meet the requirements of design inputs.
Definition of Validation
The FDA defines process validation in 21 CFR 820.3(z)(1) as follows:
“Establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.”
ISO 13485:2016, Section 7.5.6 “Validation of processes for production and service provision,” also specifies similar requirements.
Specifically, validation means:
- Demonstrating that products conforming to design specifications can be manufactured in the future
- Demonstrating that processes can be controlled as specified
- Establishing that products consistently conforming to specifications can be manufactured
Validation is a preventive activity performed before process execution, establishing that the process can consistently manufacture products meeting requirements.
Essential Differences Between Validation and Verification
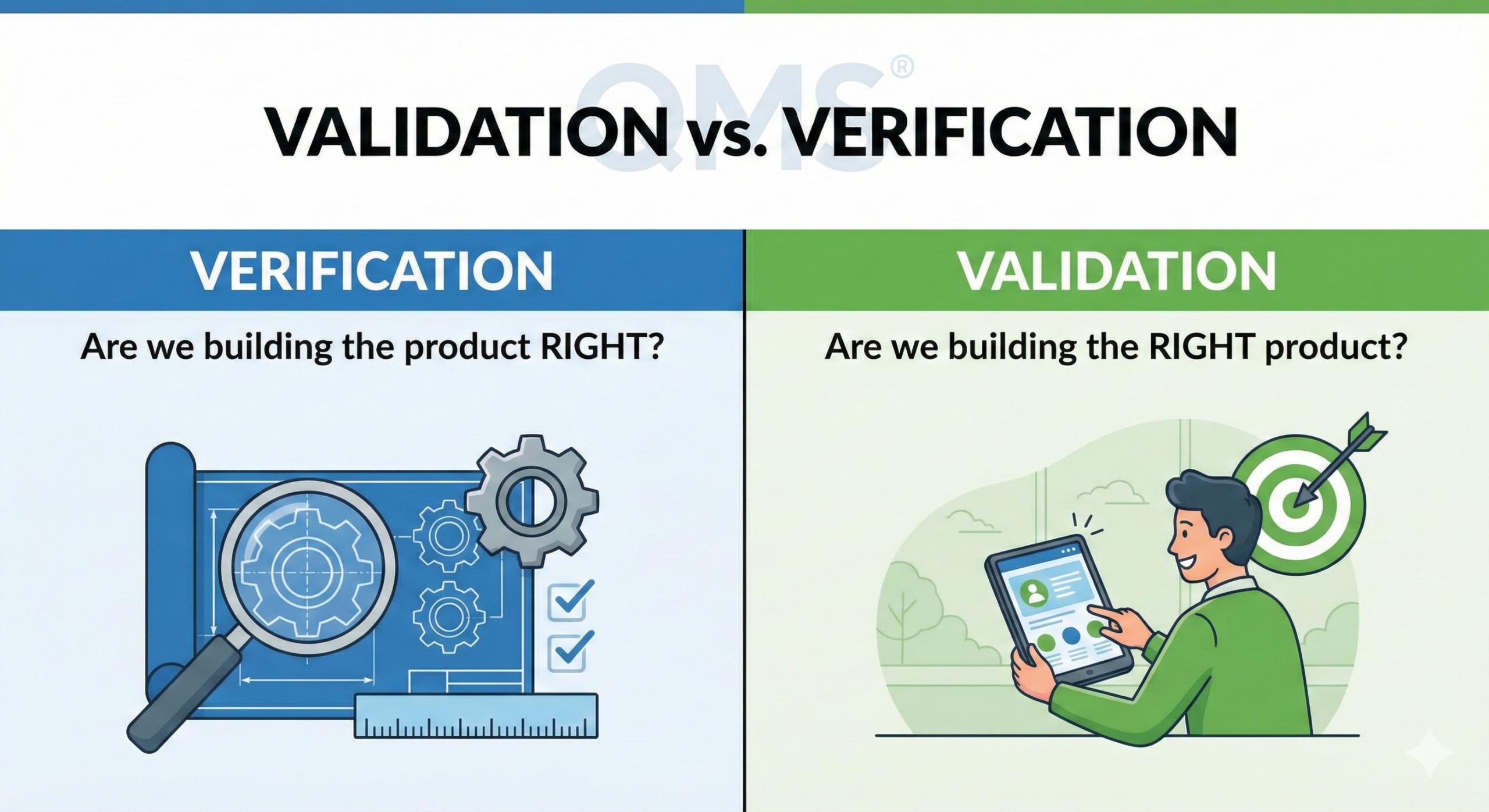
To understand the essential differences between the two, it is helpful to consider them from the perspective of tense.
| Item | Verification | Validation |
| Tense | Past tense | Future tense |
| Timing | After manufacturing | Before manufacturing |
| Subject of confirmation | Manufactured products | Process capability |
| Purpose | Confirming conformity to requirements | Establishing continuous conformity |
| Implementation method | Confirmation through inspection and testing | Systematic evaluation including statistical methods |
In other words, “validation” is a future-tense activity, while “verification” is a past-tense activity. Understanding this difference clarifies how to distinguish between the two.
Common Objective
However, in either case, the purpose is to protect patients (safety) and assure product quality. In other words, the ultimate objectives of validation and verification are the same: to assure product quality and ensure patient safety. Whether validation is performed in advance or verification is performed afterward, it is necessary to provide a high level of assurance for the quality of products being or having been manufactured and to ensure patient safety.
When Validation Is Required
What Is a Special Process?
FDA 21 CFR 820.75(a) states:
“Where the results of a process cannot be fully verified by subsequent inspection and test, the process shall be validated with a high degree of assurance and approved according to established procedures.”
So, when is validation necessary? Validation is required primarily in the following situations:
- Processes requiring destructive testing: When testing renders the product unusable
- Processes that cannot be sufficiently verified in subsequent operations: When process outputs cannot be completely confirmed non-destructively
- Processes where defects become apparent only after product use or service delivery: When the presence or absence of defects cannot be determined at the time of manufacturing
Such processes are called “special processes.”
Limitations of Destructive Testing and Problems with Sampling Inspection
Why is validation essential for processes that can only be subjected to destructive testing? The reason is that performing destructive testing makes it impossible to ship the product. Therefore, sampling inspection must be implemented.
However, sampling inspection has fundamental limitations. For example, suppose 10,000 potato chips are manufactured. Thirty of them are sampled and inspected for foreign matter. Suppose no foreign matter is found in any of them. However, the possibility that foreign matter has been mixed into any of the remaining 9,970 cannot be completely denied. This is the fundamental problem with sampling inspection.
Therefore, it is necessary to perform validation in advance and establish that the process can consistently prevent foreign matter contamination. While sampling inspection can only provide statistical assurance, validation of the process capability itself achieves a higher level of assurance.
Differences Between Process Industries and Discrete Industries
Validation in Process Industries
In general, in process industries such as pharmaceutical factories, destructive testing or sampling inspection is fundamental, so validation is essential for almost all processes. In the pharmaceutical industry, ensuring product homogeneity is important, and management of process parameters is central to quality assurance.
Characteristics of process industries:
- Primarily batch manufacturing
- Complete inspection of intermediate products is difficult
- Quality control centered on process parameters
- Validation is the primary means of quality assurance
Verification in Discrete Industries
However, in discrete industries such as medical device companies, verification is central. This is because non-destructive testing is possible for many medical devices.
Examples include:
- Measurement of electrical characteristics using oscilloscopes
- Functional confirmation using testers
- Confirmation of internal structure through X-ray fluoroscopy
- Confirmation of mechanical characteristics through dimensional measurement
Since all of these can be performed without destroying the product, 100% inspection is possible. Therefore, manufactured products (including intermediate products) can be verified, and if any non-conforming products (defective products) are found, they can be discarded or reworked (reprocessed or remanufactured). Then, only good products can be shipped.
Special Processes in Medical Devices
However, even in medical devices, there are processes that involve destructive testing or cannot be sufficiently verified in subsequent operations. FDA guidance documents and international standards cite the following as examples of special processes:
Heat treatment-related processes:
- Sterilization processes (steam sterilization, ethylene oxide sterilization, radiation sterilization, etc.)
- Heat treatment
Joining processes:
- Soldering
- Welding
- Crimping
- Bonding/Gluing
- Compression bonding
Surface treatment processes:
- Plating
- Anodizing
- Painting/Coating
Molding processes:
- Injection molding
- Aseptic filling processes
Sterile barrier systems:
- Sealing process for sterile packaging
- Selection and verification of packaging materials
For example, whether bonding has been sufficiently achieved cannot be determined by visual inspection, so destructive testing must be performed. To measure bonding strength, it is necessary to pull and destroy the bonded part. Therefore, sampling testing is unavoidable.
Similarly, for soldering, while appearance inspection can make some determination, destructive testing may be necessary to completely confirm the internal bonding condition and mechanical strength.
Thus, processes that cannot be sufficiently verified in subsequent operations are called special processes. For special processes, validation must be performed in advance to provide a high degree of assurance in advance that process outputs (intermediate products, products, etc.) meet specifications.
Methods of Validation Implementation
Three-Stage Approach: IQ, OQ, PQ
Process validation is generally implemented in the following three stages:
1. Installation Qualification (IQ)
- Confirms that equipment is correctly installed according to design specifications and manufacturer recommendations
- Verifies that utilities such as electricity, air pressure, and environmental conditions are appropriate
- Confirms that necessary documentation (operating manuals, maintenance records, etc.) is complete
2. Operational Qualification (OQ)
- Confirms that equipment functions appropriately within the specified operating range
- Verifies the setting range of process parameters
- Confirms the functions of control systems and alarm systems
- Verifies operation under worst-case conditions
3. Performance Qualification (PQ)
- Demonstrates that the process can consistently manufacture products meeting specifications under actual manufacturing conditions
- Verification with three or more consecutive lots is generally recommended
- Evaluation of process capability using statistical methods (Cpk, Ppk, etc.)
- Confirmation of long-term stability
Validation Protocols and Reports
When performing validation, the following documents must be created:
- Validation Plan: Describes the overall approach and strategy
- Protocol: Specifies concrete test methods, acceptance criteria, and sample sizes
- Validation Report: Summarizes implementation results and demonstrates that the process is qualified
Revalidation
Process validation is not a one-time activity. Revalidation is necessary in the following cases:
Revalidation based on change control:
- Changes to critical process parameters
- Equipment changes or modifications
- Changes in raw materials or component suppliers
- Changes in manufacturing location
- Occurrence of significant deviations or non-conformities
Periodic revalidation:
- For particularly critical processes (such as sterilization), periodic revalidation every 2-3 years is recommended even if no problems have occurred
- Risk assessment based on continuous process monitoring data
Continued Process Verification
ISO 13485:2016 and FDA guidance require continuous monitoring of processes after validation to maintain control status. This includes:
- Continuous monitoring of process parameters
- Application of statistical process control (SPC)
- Trend analysis of quality indicators
- Periodic review and improvement activities
Alignment with International Standards
ISO 13485:2016
Section 7.5.6 of ISO 13485:2016 specifies validation of processes for production and service provision. Process validation is required when the following conditions are met:
- Process outputs cannot be verified by subsequent monitoring or measurement
- As a result, deficiencies become apparent only after the product is used or the service is delivered
Additionally, validation is always required for the following processes:
- Sterilization processes
- Sterile barrier systems
- Computer software used in manufacturing or service provision
GHTF/IMDRF Guidance
GHTF SG3/N99-10:2004 (currently managed by IMDRF) provides comprehensive guidance on process validation. This guidance defines process validation as follows:
“A term used in the medical device industry to indicate that a process has been subject to such scrutiny that the result of the process can be practically guaranteed.”
Conclusion
Validation and verification play different but complementary roles in medical device quality assurance. Verification is a past-tense activity that confirms “whether the product was built correctly,” while validation is a future-tense activity that establishes “whether the process can consistently continue to produce correct products.”
For special processes, validation is essential due to the limitations of destructive testing and the statistical constraints of sampling inspection. On the other hand, for processes where non-destructive testing is possible, quality can be assured through 100% inspection by verification.
Whichever approach is adopted, the ultimate objective is to ensure patient safety and assure product quality. Although approaches differ between process industries and discrete industries, it is important to select appropriate control methods on a risk basis and to implement continuous improvement.
Medical device manufacturers need to understand FDA 21 CFR Part 820, ISO 13485:2016, and other applicable standards and regulatory requirements, and establish validation strategies appropriate for their products and processes. Additionally, validation is not a one-time activity but should be continuously implemented and maintained throughout the product lifecycle.

Comment