Fundamental Problems Facing Pharmaceutical Regulation
In pharmaceutical regulation, regulatory authorities and pharmaceutical companies are constantly required to strike a difficult balance.
Strengthening regulatory requirements certainly improves patient safety. However, the cost is significant. Companies must conduct more tests, prepare more detailed documentation, and build more complex quality management systems to comply with stringent regulations. These compliance costs are ultimately passed on to drug prices, becoming an economic burden on patients.
On the other hand, if regulations are relaxed, development costs decrease and drug prices can be controlled. However, if quality control standards decline, the risk of problems such as impurity contamination, lack of sterility, and insufficient content of active ingredients increases, threatening patients’ health and lives.
Limitations of Uniform Regulation
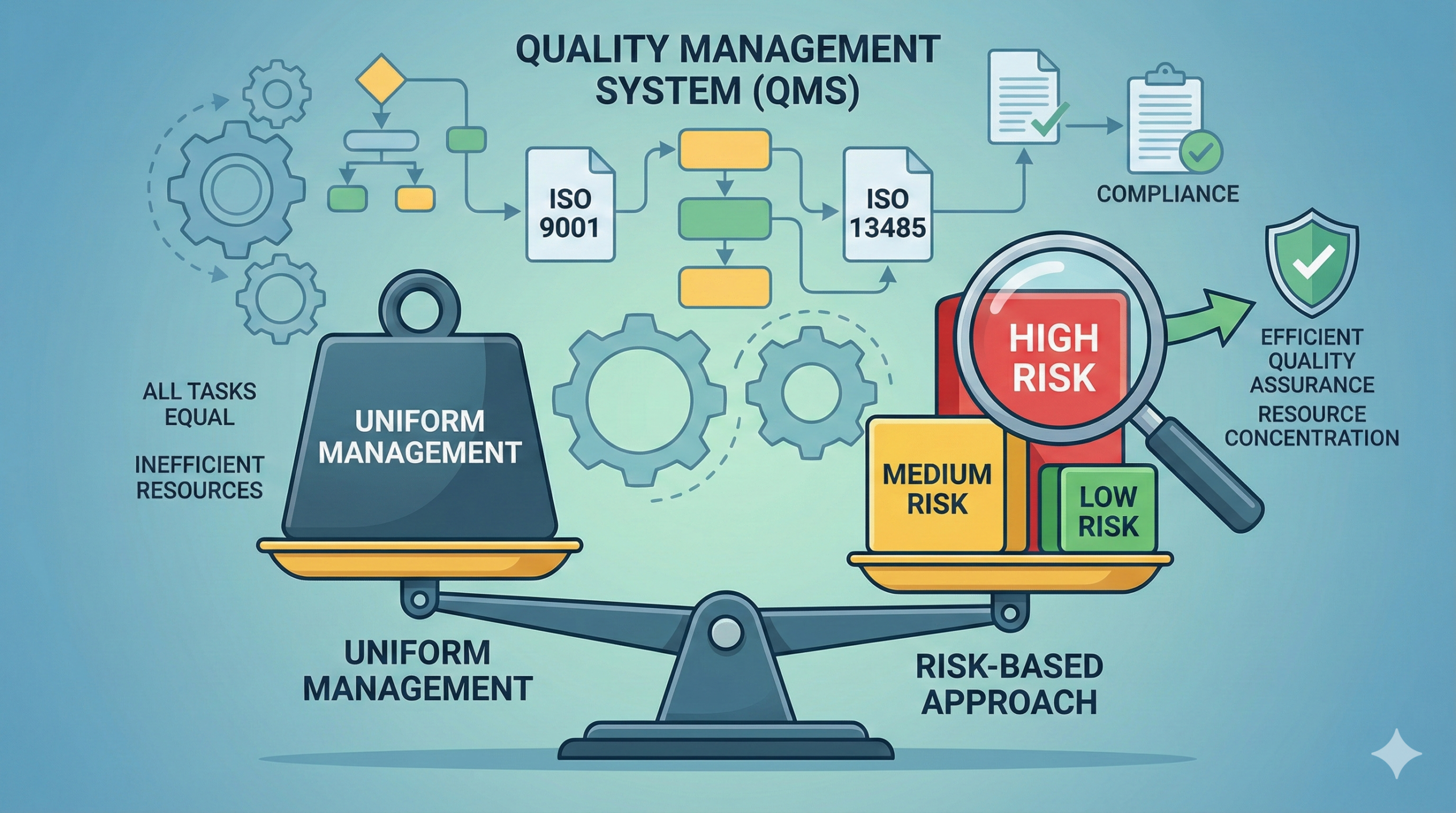
Traditional regulation was characterized by “uniformity.” Cold medicines and anticancer drugs, vitamin supplements and heart disease treatments—all were required to maintain the same level of strict management. However, this was clearly inefficient.
For example, if the color of a vitamin tablet is slightly different, there is almost no health damage to patients. However, if the active ingredient of a medicine taken by a heart disease patient is out of specification, it becomes a critical situation involving life and death. Nevertheless, requiring the same level of quality control for both represents a waste of limited resources.
Birth and Development of the Risk-Based Approach
To solve this problem, the U.S. Food and Drug Administration (FDA) launched the “21st Century cGMPs (Current Good Manufacturing Practices) Initiative” in 2002. Then in 2003, it formally proposed the “risk-based approach.”
The core of the risk-based approach is the concept of “changing the intensity of management according to the magnitude of risk.” By applying strict management to high-risk items and appropriate management to low-risk items, efficient quality assurance can be achieved.
This concept spread rapidly around the world. In 2005, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) published a guideline called “ICH Q9: Quality Risk Management,” and the risk-based approach became an international standard. Subsequently, ICH Q9 was revised in 2023 (ICH Q9(R1)) to incorporate advances in risk management practices and provide clearer guidance on the application of quality risk management principles throughout the product lifecycle.
Practical Methods for Risk Assessment
So how is risk actually assessed? Generally, the following three elements are comprehensively considered:
Severity (Magnitude of Impact) – If a problem occurs, what degree of health damage will occur to patients? For example, if bacteria contaminate a sterile injection, it can cause sepsis and threaten life, but if the imprint on a tablet is faint, there is no impact on health.
Probability of Occurrence – What is the likelihood that the problem will actually occur? This is determined from past performance, complexity of manufacturing processes, characteristics of raw materials used, and other factors.
Detectability – If a problem occurs, what is the likelihood of discovering it? The accuracy of testing methods, testing frequency, possibility of visual confirmation, and other factors are considered.
These are combined to determine the overall risk level. For example, “Severity: High” × “Probability of Occurrence: High” × “Difficult to Detect” = “Highest Risk,” requiring the most stringent management.
The most common risk assessment tool used in practice is the Failure Mode and Effects Analysis (FMEA), which systematically evaluates potential failure modes. The Risk Priority Number (RPN) is calculated by multiplying severity, occurrence, and detectability scores. Other tools include Fault Tree Analysis (FTA), Hazard Analysis and Critical Control Points (HACCP), and Hazard Operability Analysis (HAZOP).
Specific Application Examples
Let’s look at application examples of the risk-based approach.
Example of High-Risk Product: Sterile Injectables
- Contamination directly threatens patients’ lives
- Ensuring sterility is technically difficult
- Bacterial contamination cannot be detected visually
→ Apply the most stringent management: manufacturing in cleanrooms, frequent environmental monitoring, sterility testing of all lots
Example of Low-Risk Product: Vitamin Supplements
- Even with some quality variation, health damage is minor
- Manufacturing process is relatively simple
- Many problems can be detected through visual inspection
→ Standard manufacturing management and regular quality testing are sufficient
Application to Inspections
The risk-based approach has also transformed the inspection methods of regulatory authorities. Previously, all manufacturing sites were inspected at regular intervals, but now the frequency and depth of inspections vary according to risk.
For example:
- Facilities with past serious deviations
- Facilities manufacturing high-risk products such as sterile preparations
- Facilities that have introduced new manufacturing technologies
These receive more frequent and detailed inspections. Meanwhile, manufacturing sites for low-risk products that have been operating without problems for many years may have their inspection intervals extended.
The FDA’s Site Selection Model, implemented in 2015 and continuously refined, uses a data-driven algorithm to prioritize inspection sites based on multiple risk factors including product type, facility history, time since last inspection, and manufacturing complexity. Similarly, the European Medicines Agency (EMA) employs risk-based inspection planning through its coordinated Good Manufacturing Practice (GMP) inspection program.
Benefits to Companies and Patients
With the introduction of the risk-based approach, pharmaceutical companies can obtain the following benefits:
- Resources can be concentrated on important risks
- Unnecessary testing and documentation can be reduced
- Overall compliance costs can be reduced
These cost reductions ultimately lead to suppression of drug prices and reduce the economic burden on patients. At the same time, because sufficient resources are devoted to truly important risks, the safety of pharmaceuticals actually improves.
It should be noted that regulatory authorities emphasize that risk-based approaches should not be misinterpreted as reducing quality standards. Rather, they represent a more scientific and efficient allocation of quality assurance resources. The principle of “fitness for intended use” remains paramount—every pharmaceutical product must meet its quality attributes regardless of its risk classification.
Integration with Digital Technology
In recent years, the risk-based approach has undergone new evolution. With the utilization of artificial intelligence (AI) and big data analysis, more sophisticated risk prediction has become possible.
For example, by analyzing past quality data, manufacturing conditions, raw material characteristics, and other factors using machine learning, it is possible to predict in advance the possibility of quality problems occurring. This allows preventive measures to be taken before problems occur.
The concept of Pharmaceutical Quality System (PQS) 4.0, which integrates Industry 4.0 technologies, is gaining attention. Real-time monitoring using Internet of Things (IoT) sensors, predictive analytics through AI, and digital twins that simulate manufacturing processes are revolutionizing risk management. These technologies enable continuous verification of product quality rather than traditional end-product testing, embodying the shift from “quality by testing” to “quality by design.”
Furthermore, blockchain technology is being explored for ensuring data integrity in risk management documentation, and advanced process analytical technology (PAT) allows for real-time monitoring of critical quality attributes during manufacturing.
Future Prospects and Challenges
The risk-based approach has become completely established as a fundamental principle in pharmaceutical quality assurance. However, challenges remain.
Standardization of risk assessment is one such challenge. If risk assessment methods differ among companies, regulatory consistency cannot be maintained. Therefore, regulatory authorities in various countries are cooperating to develop common standards for risk assessment.
Additionally, with the emergence of new therapeutic methods (gene therapy, cell therapy, etc.), areas have appeared where traditional risk assessment methods cannot respond. Development of risk assessment techniques corresponding to these new technologies is also urgent.
The ICH is currently developing several guidelines that incorporate risk-based approaches more explicitly. ICH Q12 (Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management) and ICH Q13 (Continuous Manufacturing of Drug Substances and Drug Products) both emphasize risk-based strategies for managing changes and ensuring quality throughout the product lifecycle.
For advanced therapy medicinal products (ATMPs), including gene and cell therapies, regulatory agencies are developing specialized risk assessment frameworks. The variability inherent in these biological products, the complexity of their manufacturing processes, and their personalized nature require novel approaches to risk management that go beyond traditional pharmaceutical paradigms.
Another emerging challenge is the harmonization of risk-based approaches across different regulatory jurisdictions. While ICH guidelines provide a foundation, their implementation and interpretation can vary significantly among regions, creating challenges for global pharmaceutical companies.
Comparison of Risk Management Tools
| Tool | Primary Application | Advantages | Limitations |
|---|---|---|---|
| FMEA (Failure Mode and Effects Analysis) | Process and product design | Systematic and comprehensive; widely accepted in industry | Time-consuming; requires experienced team; subjective scoring |
| HACCP (Hazard Analysis and Critical Control Points) | Manufacturing process control | Focus on critical control points; preventive approach | Originally designed for food industry; may need adaptation |
| FTA (Fault Tree Analysis) | Complex system analysis | Good for identifying root causes; graphical representation | Complex to construct; requires detailed system knowledge |
| Risk Ranking and Filtering | Prioritization of risks | Quick and simple; useful for initial assessment | Less detailed than other methods; may oversimplify complex risks |
| Preliminary Hazard Analysis | Early design stages | Identifies hazards early; cost-effective | Less detailed; requires follow-up with other tools |
Evolution of Regulatory Guidance
| Year | Event | Significance |
|---|---|---|
| 2002 | FDA launches 21st Century cGMPs Initiative | Beginning of modern risk-based regulation |
| 2003 | FDA formally proposes risk-based approach | Official recognition of risk-based methodology |
| 2005 | ICH Q9 published | International harmonization of quality risk management |
| 2006 | ICH Q8 (Pharmaceutical Development) | Introduction of Quality by Design (QbD) concept |
| 2009 | ICH Q10 (Pharmaceutical Quality System) | Integration of risk management into quality systems |
| 2012 | ICH Q11 (Development and Manufacture of Drug Substances) | Application to active pharmaceutical ingredients |
| 2015 | FDA implements risk-based site selection model | Data-driven inspection prioritization |
| 2019 | ICH Q12 finalized | Lifecycle management with risk-based approaches |
| 2023 | ICH Q9(R1) published | Updated guidance incorporating lessons learned |
Conclusion
The seemingly contradictory goals of “ensuring safety” and “controlling costs” in pharmaceutical regulation—the risk-based approach has provided a realistic solution to this eternal dilemma.
Rather than managing everything uniformly, the intensity of management is changed according to risk. Through this simple but innovative concept, it has become possible to allocate limited resources to where they are most needed and provide patients with safer and more affordable pharmaceuticals.
The risk-based approach is not merely a regulatory technique. It is a new paradigm for pharmaceutical quality assurance that combines scientific thinking with realistic judgment. There is no doubt that it will continue to evolve with technological advances and contribute to improving the quality of healthcare.
As we move forward, the integration of advanced technologies such as AI, machine learning, and real-time monitoring systems will further enhance our ability to identify, assess, and mitigate risks. The convergence of regulatory science and data science promises to make risk-based approaches even more predictive and preventive, ultimately benefiting both the pharmaceutical industry and, most importantly, the patients who depend on safe and effective medicines.
The future of pharmaceutical regulation lies not in choosing between safety and efficiency, but in achieving both through intelligent, science-based risk management. This represents not just a change in how we regulate, but a fundamental shift in how we think about quality in the pharmaceutical industry.

Comment