The Birth of the Validation Concept The idea of validation was inspired by the drug-related deaths caused by large-volume injectable drugs in the United States in the 1970s.
The injectable drug in question was sterilized during the manufacturing process and conformed to sterility testing during shipment testing.
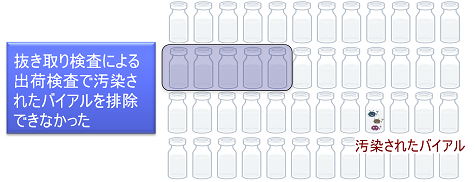
The manufacturer shipped the product after confirming that it conformed to testing, but a series of accidents occurred in which patients who were administered this product died.
The cause of the accident was that water contaminated with bacteria was used as cooling water, which caused the inside of the heat-sterilized vial to depressurize, and the contaminated water passed through the gap between the vial and the rubber stopper, contaminating the content liquid.
The shipping test was a sampling test for sterility, which was not conducted on the entire number of vials, and the presence of contaminated vials could not be detected.
From this accident, the U.S. FDA realized the need to not only focus on the quality of the final product, but also to ensure that quality is ensured in the manufacturing process, and decided to incorporate the concept of validation into its regulations.
related product
[blogcard url=https://xn--2lwu4a.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ] [blogcard url=https://xn--2lwu4a.jp/qms-rx/ title=”QMS(手順書)ひな形 医薬品関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/O006.html title=”【VOD】具体的な事例で学ぶGMP実践セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-034.html title=”【セミナービデオ】最終的に滅菌される医療機器の包装 ISO 11607-1,-2 2019年版への対応セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/O095.html title=”【VOD】医療機器における洗浄および滅菌バリデーション・包装滅菌バリデーションの進め方”] [blogcard url= https://ecompliance.co.jp/SHOP/QMS-MHLW-28.html title=”【2021年度改正QMS省令対応】プロセスバリデーション規程・手順書・様式”]]]>


Comment