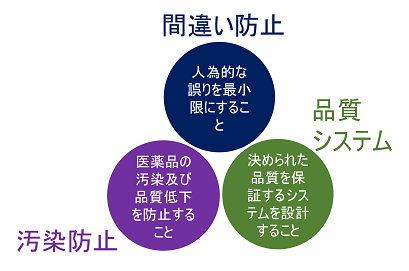
Three principles of GMP
The three principles of GMP are as follows
- Minimize human error
- Prevent contamination and quality loss
- Design systems that assure a higher level of quality
These are well-known terms, but it is not known who actually originated them. They are not mentioned in any regulatory requirements or notices. They seem to have been used to provide an easy-to-understand explanation when GMP was introduced in Japan.
The three principles of GMP vary from country to country.
For example, in the U.S. FDA,
- keep it clean
- check and double check
- write it and down
It is considered to be a
On the other hand, the three principles in the WHO-GMP are,
- Reduction of human error (rules, training)
- Prevent contamination and quality loss
- Building a system to enhance quality
The first is.
From this, it can be inferred that the three principles of GMP in Japan are based on WHO.
What are the three principles of GMP in Japan intended to be?
1. Minimize human error
Standardization of work through documentation (SOP), education and training, etc.
2.Prevent contamination and quality loss
prevention of cross-contamination
Cleaning, line clearance, equipment maintenance, etc.
3 Design systems that assure a higher level of quality
Deviation and change management, product quality verification, management review, etc.
The first is.
The U.S. FDA was the first in the world to legislate GMP in 1963. This was triggered by the thalidomide incidents that occurred between 1960 and 1962.
In 1969, the WHO established GMP and recommended that member countries adopt and implement a certification system based on GMP in the trade of medicinal products.
In Japan, the Ministry of Health and Welfare (MHW) first instructed pharmaceutical manufacturers to implement GMP in 1974 in its “Standards for the Manufacture and Quality Control of Pharmaceuticals” (MHW Pharmaceutical Affairs Bureau Director-General Notification YAKUHAKU No. 801). The WHO GMP was probably referred to at that time.
related product
[blogcard url=https://xn--2lwu4a.jp/qms-rx/ title=”QMS(手順書)ひな形 医薬品関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/O006.html title=”【VOD】具体的な事例で学ぶGMP実践セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/P170_EP170a.html title=”【書籍】【信頼性基準適用試験/GLP/GMP】データインテグリティに適合するための電子/紙データ・記録の運用管理とSOP作成手法”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-109.html title=”【セミナービデオ】データインテグリティの具体的な手順書作成セミナー 【データインテグリティ書籍付】”] [blogcard url= https://ecompliance.co.jp/SHOP/O019.html title=”【VOD】改正GMP省令対応の「管理監督者のGMP教育」/「企業文化」の見直し醸成セミナー”]]]>


Comment