Importance of Cleaning Validation
In order to guarantee sterilization of sterilized medical devices, it is necessary to ensure that residual foreign substances do not affect the human body.
For this purpose, it is important to decompose and remove adhered materials as much as possible by cleaning.
It is essential to verify in advance that the cleaning process established by the cleaning validation can achieve the intended cleaning effect, and to ensure that the cleaning process is implemented in the field.
In particular, “remanufactured single-use devices” (R-SUDs) are disassembled, cleaned, and reassembled to properly remove contamination during use at medical institutions, and then re-distributed as SUDs with the same quality, efficacy, and safety as the original medical devices.
In the remanufacturing of SUDs, appropriate cleaning, disinfection, and sterilization methods are required for the target R-SUDs, and validation of the cleaning process is required.
The purpose of cleaning medical device products is to remove residues/contaminants/microorganisms, etc.
The following two types of medical devices require cleaning
1.When the product is sterilized or cleaned by a tissue prior to its use
2.When the product is supplied non-sterile and sterilized or cleaned prior to its use
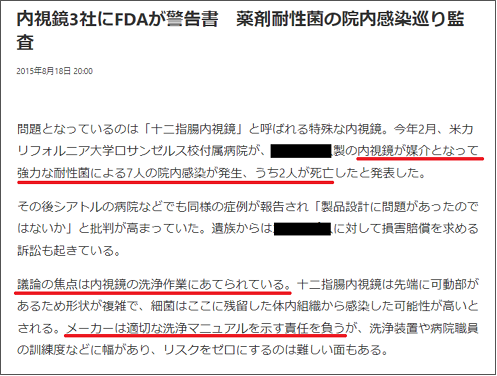
In 2015, the FDA issued a warning letter to three Japanese endoscope companies. This was due to an endoscope-borne outbreak of seven nosocomial infections in the U.S. caused by a strongly resistant bacterium, two of which resulted in death.
The discussion focused on the cleaning process of the endoscope. Duodenoscopes have a complex shape due to the movable tip, and it is highly likely that bacteria were transmitted from internal tissues that remained in the endoscope. Manufacturers are responsible for providing appropriate cleaning manuals, but there is a wide range in cleaning equipment and the level of training of hospital personnel, making it difficult to eliminate the risk. The FDA stated in the letter, “We are aware that there have been cases of infection even when manuals are strictly followed.
The requirements for information that medical device companies must provide to medical institutions regarding cleaning, etc., are summarized in ISO 17664-1:2021, “Processing of healthcare products – Information to be provided by manufacturers of medical devices for the processing of medical devices”.
The instruction manual must include precautions for cleaning/disinfection/sterilization, etc.
When cleaning methods such as automatic cleaning, manual cleaning, etc. are implemented in a healthcare facility, cleaning validation must be performed to ensure that a specific level of cleanliness is guaranteed.
Automatic hand washing is desirable because of the risks associated with hand washing, including cut wounds and exposure of medical personnel to blood and body fluids.
It is also effective in preventing infection.
The entity that performs the cleaning is,
1.Medical device manufacturers
2.Remanufactured medical device manufacturer
3.medical institution
classified as follows.
1.Cleaning validation by medical device manufacturers
Medical device manufacturers must perform cleaning validation as required.
However, there is no direct requirement for cleaning validation in ISO 13485:2016.
In ISO 13485:2016 “Practical guide”, the cleaning process is considered “a process that should be individually determined whether or not validation should be performed”.
ASTM F3127-16 “Standard Guide For Validating Cleaning Processes Used During The Manufacture Of Medical Devices” is the only guideline. It is also a Recognized Consensus Standard of the FDA.
However, ASTM F3127-16 does not cover reusable medical devices (i.e., those cleaned in medical facilities).
On the other hand, cleaning must be performed prior to sterilization to ensure sterilization and reduce sterilization time.
2.Cleaning validation by the remanufactured medical device manufacturer
In the case of “remanufactured single-use medical devices” (R-SUDs), cleaning validation is more important than for original medical devices because they are contaminated with blood, body fluids, etc.
The 2021 revision of the QMS Ministerial Ordinance added Chapter 5-2 “Manufacturing Control and Quality Control of Remanufactured Single-Use Medical Devices” (Articles 81-2 to 81-2-4).
The QMS Ministerial Ordinance requires cleaning validation of the remanufacturing process for “remanufactured single-use devices” (R-SUD).
Guidelines for cleaning validation of “remanufactured single-use medical devices” (R-SUDs) include the following
1.Cleaning Guidelines and Questions and Answers (Q&A) for Businesses Concerning Remanufactured Single-Use Medical Devices (Office Communication dated June 17, 2048)
2.Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling (Final Change of Schedule: June 9, 2017)
3.Implementation of cleaning validation by medical institutions
Perform manual cleaning, immersion cleaning, ultrasonic cleaning, and washer-disinfector cleaning.
In many cases, cleaning and disinfection are used to detoxify the bacteria, and it is not always possible to carry out the process to sterilization. The situation is such that high-level disinfection, rather than sterilization, is acceptable for the time being.
Due to aging equipment and other factors, gaps, cracks, and other problems occur that prevent complete cleaning.
Japanese versions of the guidelines established by the German Society for Sterilization (DGSV) and other organizations exist.
1.Validation Guidelines for Hand Washing and Hand Disinfection of Medical Devices
2.Guidelines for the validation and routine monitoring of automatic cleaning and hot water disinfection processes for medical devices
PQ in sterilization validation and cleaning validation is required in the medical field.
related product
[blogcard url=https://ecompliance.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ]]]>


Comment