Use Error and Human Error: Understanding the Critical Distinction
Introduction: Deep Understanding of Use Error and Human Error
Is it simple to master the tools and systems we encounter in daily life? Or do we find ourselves struggling with the challenges posed by technology in more specialized domains? Particularly in the context of medical devices, pharmaceuticals, and other safety-critical products and systems, the occurrence of errors has direct implications for patient safety. This article clarifies two frequently misunderstood concepts: “use error” and “human error.” A precise understanding of these error classifications is essential for building a robust quality culture throughout the medical device industry, from product development to post-market surveillance.
Is it simple to master the tools and systems we encounter in daily life? Or do we find ourselves struggling with the challenges posed by technology in…
Defining Use Error
To accurately understand the definition of “use error,” it is necessary to consult international standards and regulatory guidance. According to the United States FDA (Food and Drug Administration) guidance and IEC 62366-1 (Medical Devices – Application of Usability Engineering to Medical Devices), use error is defined as follows:
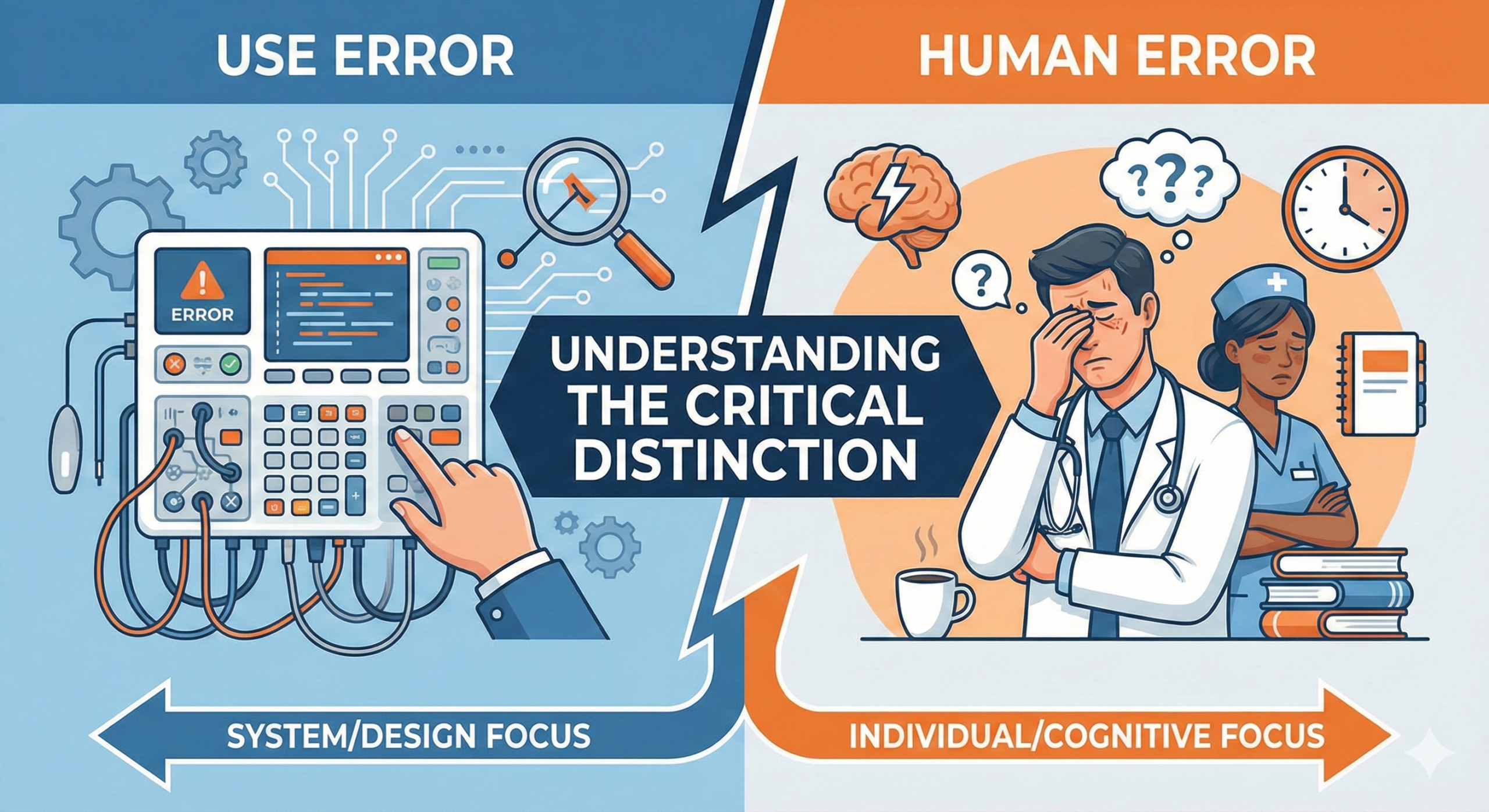
A use error is an action performed by a user of a medical device or system that fails to achieve the intended objective, and as a result, may cause harm to a patient. The critical point in this definition is that it does not attribute the cause to simple factors such as “user carelessness” or “insufficient training.” Rather, it highlights a fundamental design issue: the product or system design, user interface, labeling, warnings, or instructions for use do not align with user expectations or capabilities.
More specifically, use errors possess the following characteristics. First, they are predictable. By conducting appropriate usability evaluation during the design phase, designers can prospectively identify scenarios in which misoperation is likely to occur. Second, responsibility for use errors lies primarily with the design team and manufacturer, since users attempt to operate products in a “reasonable manner.” Third, use errors are phenomena that occur not only during the design phase but also after the device reaches the market. Therefore, medical device manufacturers have an obligation to continuously monitor use errors in the post-market context and implement corrective actions as necessary.
Concrete Examples of Use Error in Medical Devices
To illustrate use error in the medical device context, consider an insulin pen. When a patient scrolls a dial to set the insulin dose, if the dose markings lack sufficient visibility, the patient may inadvertently set the dose to 2 units instead of the intended 20 units. In this case, the patient acted in a “reasonable manner” when attempting to use the product, but the device’s user interface design was insufficient, resulting in an error. This is a paradigmatic example of use error.
Another illustrative example is an infusion pump. In a system that requires users to navigate multiple input screens to set the medication infusion rate, users may become confused by the operational differences between screens and inadvertently input an unintended rate. Such instances are also classified as use errors. In these examples, healthcare professionals received appropriate training and did not intentionally commit errors. Rather, the product design or user interface failed to adequately account for user expectations and cognitive load, making these failures fundamentally attributable to design deficiencies rather than user incompetence.
Defining Human Error
In contrast, “human error” is a considerably broader concept. Human error refers to a class of phenomena in which the cognitive, physical, or behavioral limitations of human beings result in failure to execute an intended action, ultimately producing an undesired outcome. According to international standards such as ISO 14971 (Risk Management for Medical Devices) and IEC 62304 (Lifecycle Processes for Medical Device Software), human error encompasses multiple subcategories.
Human errors manifest in several distinct forms. “Slip errors” occur when an individual omits an essential step, either intentionally or unconsciously. “Execution errors” occur when an individual possesses the correct intention to perform a step but, due to physical difficulty or inattention, performs an unintended action. “Planning errors” result from insufficient comprehension of the overall procedure, leading to action based on an inadequate plan.
Human errors are influenced not only by individual intent or negligence but also by organizational context, fatigue, stress, time pressure, insufficient training, or inadequate standard operating procedures (SOPs). In the medical device industry, these contributing factors are often referred to as “latent failures,” and their mitigation requires system-level organizational improvements.
Concrete Examples of Human Error in the Medical Device Industry
A concrete example within the medical device industry illustrates human error in manufacturing quality control. During the manufacturing process of a medical device, a production worker may, despite understanding the criticality of component inspection, unconsciously omit several inspection steps due to time pressure and fatigue on the production line. Although the worker received appropriate training and the device possesses no design defects, the problem lies in the interaction between human cognitive and physical limitations and organizational environment (time pressure). This exemplifies a typical human error scenario.
Another case study concerns pharmacy practice in a clinical setting. When a pharmacist reviews multiple patient prescriptions simultaneously, the cognitive demand of context-switching may lead to confusion between Patient A’s and Patient B’s prescriptions, resulting in the incorrect medication being dispensed to a patient. Although the pharmacist received adequate training, and the error reflects some degree of inattention, the root cause is not a personal character deficiency but rather the cognitive burden of the work environment. Accordingly, the organization must not only hold the individual accountable but also implement organizational improvements such as workflow optimization, enhanced verification systems, or appropriate workload management.
The Essential Distinction Between Use Error and Human Error
The differences between these two concepts are clarified in the following table.
| Characteristic | Use Error | Human Error |
| Definition | An action performed by a user of a medical device that results in failure to achieve the intended objective due to design or UI deficiencies, potentially causing harm | A phenomenon in which human cognitive or physical limitations result in failure to execute an intended action, producing an undesired outcome |
| Attribution of Responsibility | Product designer and manufacturer | Individual and organizational system |
| Predictability | High (prospectively identifiable through usability evaluation) | Moderate to Low (influenced by multiple factors) |
| Mitigation Strategy | Design modification, UI improvement, enhanced labeling and warnings, human factors engineering | Human error prevention measures, training, SOP improvement, organizational culture change |
| Regulatory Position | Manufacturer responsibility; post-market reporting required | Subject to risk management; organizational improvement mandatory |
Regulatory Requirements and Industry Trends
Latest FDA Guidance Developments
The United States FDA has progressively strengthened its guidance on usability engineering and human error prevention between 2023 and 2024. Notably, the “Infusion Device Improvement Initiative” strongly recommends that for complex medical devices, usability testing aimed at minimizing use errors be conducted in settings that closely approximate real-world use environments. This guidance emphasizes the importance of evaluating use scenarios in actual clinical contexts, not only in laboratory settings.
Alignment with EU MDR Regulations
The European Medical Device Regulation (EU MDR) similarly emphasizes the distinction between use error and human error. Specifically, in the preparation of the Risk Management File, manufacturers are required to conduct detailed analysis of use error scenarios anticipated in the specified conditions of use and document corresponding mitigation strategies. Experience from 2024 implementation efforts confirms that the robustness of human factors engineering (HFE) strategies has become a critical evaluation point in regulatory assessment.
Japanese PMDA Regulatory Trends
The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has also intensified its scrutiny of use error analysis and human error prevention measures in medical device approval applications. The trend from 2024 through 2025 indicates that for software-enabled medical devices and those featuring complex user interfaces, submission of a usability evaluation report based on IEC 62366-1 is becoming mandatory.
Organizational Implementation and Safety Culture
Contemporary organizational theory and medical safety research increasingly emphasize that precise understanding of these error classifications and appropriate organizational management of these phenomena through established processes and procedures are essential. As the medical device industry enters 2025, the focus extends beyond the mere implementation of quality management systems to encompass the development of a “safety culture” that prioritizes error prevention as a strategic organizational priority.
Concretely, organizations should implement the following measures. First, deliberately avoid conflating use error and human error, and develop organizational competency in appropriately addressing each. Second, prioritize design-based mitigation strategies grounded in human factors engineering for use errors. Third, for human errors, move beyond individual accountability to implement organizational environmental improvements such as clarification of standard operating procedures, enrichment of training programs, and optimization of workflow design.
Conclusion
Comprehending the essential distinction between use error and human error is prerequisite knowledge for all stakeholders in the medical device industry. When these concepts are properly understood and appropriately applied, both the safety and efficacy of medical devices improve significantly, ultimately ensuring patient safety. When all stakeholders—medical device manufacturers, regulators, healthcare institutions, and patients—operate from a shared understanding of these concepts, the reliability of medical technology and its contribution to society become assured. We sincerely hope that this article contributes meaningfully to the daily professional endeavors and technical challenges of those working in the medical device industry.

Comment