Why Medical Devices Need Usability Engineering
The Necessity of Usability Engineering in Medical Devices
In clinical settings, operational errors with medical equipment can lead to serious accidents that directly affect patient lives. Particularly for life-sustaining devices such as infusion pumps, excellent usability design is essential for ensuring patient safety.
Infusion Pump Operation Error Incidents
Background and Latest Trends
Infusion pumps are medical devices that continuously deliver precise amounts of medication or nutritional solutions to patients. However, numerous serious accidents caused by operational errors have been reported.
Latest Medical Accident Trends
In Japan’s medical accident reports for 2023, 6,070 medical accidents were reported, representing a 14.2% increase from the previous year. Of these incidents, 7.4% resulted in fatalities. Accidents caused by medical device operational errors continue to occur, and improvement through usability engineering is recognized as an urgent issue.
Typical Accident Patterns
Setting Value Input Errors
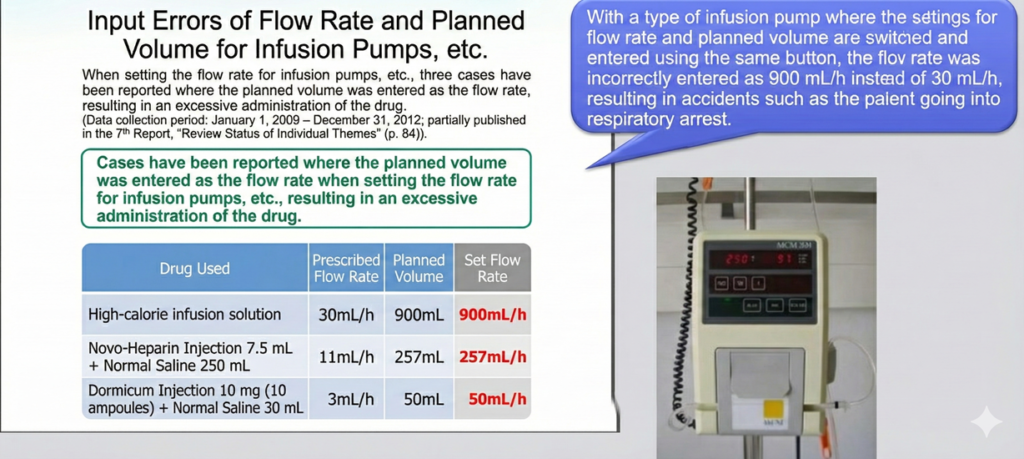
These errors occur when healthcare professionals incorrectly enter critical parameters into infusion pumps. Common mistakes include confusion between dosage rate units such as ml/h (milliliters per hour) and ml/min (milliliters per minute), which can result in administration rates that are 60 times too high or too low. Decimal point positioning errors represent another critical vulnerability, where entering 10.0 as 100 delivers ten times the intended dose. Additionally, digit errors in total infusion volume, such as setting 100ml as 1000ml, can lead to massive overdosing or underdosing situations.
Alarm Response Errors
Healthcare workers face challenges in correctly interpreting alarm meanings, particularly when multiple devices are generating alerts simultaneously. Misjudgment of urgency levels can result in delayed responses to critical situations. Furthermore, insufficient confirmation of settings after alarm cancellation often leads to continued incorrect infusion rates, compounding the original error.
Equipment Operation Confusion
In busy clinical environments where multiple infusion pumps are used simultaneously for different patients or different medications, confusion in pump settings is a recurring problem. Misreading of screen displays, especially in low-light conditions or when viewing from angles, contributes to errors. Misunderstanding of button operations, particularly when devices lack intuitive interfaces, adds another layer of risk to patient safety.
Actual Accident Cases
Real-world reported cases demonstrate the severity of these operational errors. In one incident, a flow rate that should have been set at 830mL/h was mistakenly entered as 83mL/h, delivering only one-tenth of the required medication. Another case involved a syringe pump where 4.1mL/h was incorrectly entered as 41mL/h, resulting in a tenfold overdose. Perhaps most alarmingly, unit selection errors have led to cases of 16.7-fold overdosing, where the wrong unit was selected on the device interface.
These accidents stem from unclear screen displays and ambiguous operation interfaces on the devices, seriously impacting patient safety.
Interface Design Problems
Issues in Traditional Design
Visibility Problems
Traditional medical device interfaces often suffer from fundamental visibility issues that impair safe operation. Small fonts and numbers, particularly problematic for aging healthcare workers or in emergency situations, make it difficult to confirm critical settings. Insufficient contrast between display elements and backgrounds, especially under varied lighting conditions, increases the likelihood of misreading. Important information is frequently placed in locations that are easily overlooked, such as secondary screens or corner positions, leading to missed critical alerts or settings.
Operability Problems
Design flaws in physical interaction further compound usability issues. Buttons with similar appearances but different functions can be easily confused under stress. Illogical operation sequences that don’t match users’ mental models or clinical workflows increase cognitive load and error rates. Insufficient feedback, whether visual, auditory, or tactile, leaves operators uncertain whether their inputs were successfully registered, often resulting in repeated button presses or abandonment of critical setting changes.
Deficiencies in Error Prevention Functions
Many current infusion pumps lack robust error prevention mechanisms. Inadequate detection of abnormal value inputs allows obviously incorrect settings to be accepted without challenge. Insufficient confirmation screens fail to give operators adequate opportunity to verify their entries before execution. Complicated cancellation functions make it difficult to correct errors once detected, sometimes leading operators to work around problems rather than properly rectifying them.
Increased Cognitive Load
Healthcare workers operate medical devices under challenging conditions that amplify the importance of good usability design. Time pressure in emergency situations demands immediate, confident operation without opportunity for deliberation. Simultaneous care of multiple patients divides attention and working memory, making simple, consistent interfaces essential. Emergency judgment requirements create stress that can interfere with complex operational sequences. Long working hours and resulting fatigue degrade cognitive function, making poorly designed interfaces even more hazardous.
Under these circumstances, non-intuitive operation interfaces become significant risks to patient safety.
The Importance of Usability Engineering
Human Error Prevention
Error Prevention Design
Effective usability engineering implements multiple layers of error prevention. Operation procedures are designed to make mistakes difficult to execute, incorporating constraints that guide users toward correct actions. Automatic checking functions validate abnormal values before they can be applied, alerting operators to potentially dangerous settings. Confirmation steps are optimized to balance thoroughness with efficiency, providing meaningful opportunities for verification without creating “alarm fatigue” that leads to automatic dismissal of warnings.
Error Recovery Support
When errors do occur, well-designed systems facilitate rapid recovery. Clear error messages explain what went wrong in clinical rather than technical language, helping operators understand the problem quickly. Simple correction procedures minimize the time and steps required to rectify errors, reducing the period during which patients are at risk. Display of operation history allows healthcare workers to review their actions and understand the sequence of events leading to an error, supporting both immediate correction and learning for future prevention.
Improved Usability
Intuitive Operation
Optimal medical device interfaces adopt operation patterns that healthcare workers find natural and familiar. This includes using common conventions from consumer electronics where appropriate, while recognizing the unique demands of clinical environments. Visually understandable displays utilize color, size, and position to convey information hierarchy and urgency. Logical screen transitions match clinical workflows, anticipating the next steps in common procedures and minimizing navigation requirements.
Efficiency Improvements
Usability engineering enhances clinical efficiency through thoughtful design choices. Frequently used functions are given rapid access paths, often through dedicated physical buttons or prominent screen positions. Operation steps are minimized without sacrificing safety, streamlining routine tasks while maintaining safeguards for critical actions. Personal setting storage functions allow individual healthcare workers or units to configure devices according to their preferences and protocols, reducing setup time and potential for configuration errors.
Safety Assurance
Multiple Safety Devices
Comprehensive safety requires multiple layers of protection. Functions preventing dangerous operations include mechanical interlocks, software constraints, and confirmation requirements that must be consciously overridden. Automatic stop functions detect anomalous conditions and halt infusion before harm occurs, such as air-in-line detection or occlusion pressure sensing. Abnormal value warning systems alert operators to potentially incorrect settings, implementing intelligent ranges based on clinical context and patient parameters.
Traceability
Modern medical devices must support accountability and analysis through comprehensive recording capabilities. Operation logs capture every action taken with the device, creating an audit trail for investigation of adverse events. Change history for settings documents when and by whom parameters were modified, supporting both real-time supervision and post-event review. Clarification of responsibility helps ensure proper oversight and creates accountability that encourages careful operation.
Specific Improvement Approaches
User-Centered Design (UCD)
User Research
Effective usability engineering begins with thorough understanding of actual use contexts. Observation of real use environments reveals how devices are actually employed in practice, often differing from manufacturer assumptions. Interviews with healthcare workers uncover pain points, workarounds, and latent needs that may not be apparent from incident reports alone. Task analysis implementation breaks down complex procedures into constituent steps, identifying where errors are most likely to occur and where design improvements can have the greatest impact.
Prototype Evaluation
Iterative design processes employ progressively refined prototypes to validate design decisions before expensive production commitments. Testing in simulated environments allows assessment under controlled conditions that approximate clinical reality. Usability testing implementation with representative users performing realistic tasks reveals problems that may not be apparent to designers. Repeated refinement based on test findings progressively eliminates usability issues and optimizes the user experience.
Compliance with International Standards
IEC 62366-1 (Usability Engineering for Medical Devices)
This international standard, issued as IEC 62366-1:2015+AMD1:2020, provides the primary framework for usability engineering in medical device development. Implementation of the usability engineering process it describes ensures systematic consideration of use-related risks throughout the development lifecycle. Execution of risk analysis specifically focused on use errors identifies hazards that might not be apparent through traditional safety analysis. Use error evaluation through formative and summative testing validates that risk control measures are effective in practice.
In Japan, this standard was adopted as JIS T 62366-1:2022 and published in February 2022, with compliance becoming mandatory from March 31, 2024. This regulatory requirement reflects growing recognition of usability’s importance in medical device safety. The standard’s adoption represents a significant advancement in Japan’s medical device regulatory framework, aligning Japanese requirements with international best practices and emphasizing the critical role of human factors engineering in device safety.
ISO 14971 (Risk Management for Medical Devices)
This complementary standard addresses broader risk management concerns, with usability engineering addressing the use-related subset. Identification of use-related risks considers how device interaction might lead to hazardous situations. Implementation of risk control measures addresses these risks through design improvements, protective measures, or information for safety. Verification of effectiveness ensures that risk control measures actually reduce risk to acceptable levels in real-world use.
Application of Design Principles
Visibility Principle
Effective interfaces make critical information immediately apparent. Emphasis display of important information uses size, color, brightness, and position to draw attention to what matters most. Clear display of status ensures operators always know the current state of the device and infusion. Visualization of progress helps healthcare workers understand how treatments are advancing and when intervention may be needed.
Correspondence Principle
Well-designed interfaces create clear relationships between actions and outcomes. Clear connections between operations and results help users build accurate mental models of device behavior. Logical physical arrangement places controls and displays in positions that match users’ expectations based on their function. Consistent operation methods across different functions and different devices reduce learning burden and transfer of training.
Constraint Principle
Intelligent constraints guide users toward safe operation. Prevention of inappropriate operations makes incorrect actions impossible or difficult to execute accidentally. Appropriate limitation of choices reduces cognitive load by eliminating irrelevant options based on context. Provision of guidance functions offers help at the point of need, supporting users through complex or infrequent procedures.
Implementation Effects and Results
Accident Reduction Effects
Medical devices that apply usability engineering principles demonstrate measurable safety improvements. Significant reduction in accidents caused by setting errors is consistently observed when robust error prevention and detection mechanisms are implemented. Shortened alarm response time results from clear, prioritized alerts that communicate urgency and required action effectively. Improved healthcare worker satisfaction reflects reduced frustration and increased confidence in device operation, contributing to better workplace morale and potentially to staff retention.
As a concrete example of improvement, Murata Manufacturing’s infusion controller “SEEVOL” solved conventional problems through droplet detection technology using cameras and comprehensive usability design. The device received the Good Design Award in 2022, serving as a demonstrated example of usability improvement. This recognition validates the practical benefits of applying systematic usability engineering principles to medical device development.
Economic Effects
Beyond safety improvements, usability engineering delivers economic value to healthcare institutions. Reduction of damages from medical accidents includes avoided liability costs, reduced patient harm, and preserved institutional reputation. Shortened education and training time results from more intuitive interfaces that require less extensive instruction for competent use. Improved device utilization efficiency means healthcare workers spend less time troubleshooting or working around device limitations and more time on direct patient care.
Future Challenges and Prospects
Response to New Technologies
Emerging technologies present both opportunities and challenges for usability engineering. The increase in IoT-enabled devices creates new complexity in device ecosystems, requiring consideration of system-level usability beyond individual devices. Integration of AI functions introduces questions about transparency, trust, and appropriate allocation of decision-making between human and machine intelligence. Remote monitoring systems change the context of device interaction, requiring new approaches to ensure safe operation when users and devices are physically separated.
Regulatory Strengthening
Regulatory authorities worldwide are increasing their focus on usability engineering. More stringent usability evaluation requirements reflect growing recognition of human factors’ role in device safety. Continuous improvement demands mean that demonstrating initial compliance is not sufficient; manufacturers must show ongoing attention to usability throughout the device lifecycle. International standard harmonization facilitates global market access while ensuring consistent safety levels across regions.
Importance of Education
Realizing the full benefits of usability engineering requires investment in human capital. Education for healthcare workers ensures they can effectively operate even well-designed devices and recognize when device limitations require adaptation of procedures. Skill enhancement for designers ensures the next generation of medical devices incorporates human factors considerations from the earliest concept stages. Organizational initiatives create cultures that value usability and safety, supporting the resources and commitment necessary for effective implementation.
Conclusion
Usability engineering in medical devices is not merely about improved ease of use but is a critical element directly connected to patient life safety. As demonstrated by infusion pump cases, inappropriate interface design can trigger serious medical accidents.
Through systematic usability engineering processes, it is essential to prevent human errors and build an environment where healthcare workers can operate equipment safely and efficiently. This has become an indispensable requirement in modern healthcare.
As technology continues to advance, more sophisticated and comprehensive usability engineering approaches will be demanded in the future. The integration of emerging technologies, strengthening regulatory frameworks, and growing emphasis on human factors education all point toward an evolving landscape where usability engineering plays an increasingly central role in medical device development and safe clinical practice.

Comment