What is Traceability in the V-Model?
The V-Model and Traceability: Effectiveness and Key Points for Implementation
Are you familiar with the terms “V-model” and “traceability”? Even if you have heard of them, many people may not fully understand what they specifically refer to or why they are important. This article explains these concepts in a manner that is accessible to beginners, while maintaining professional rigor. It integrates the latest regulatory developments and demonstrates practical application methods at the implementation level.
Are you familiar with the terms “V-model” and “traceability”? Even if you have heard of them, many people may not fully understand what they specifica…
What is the V-Model?
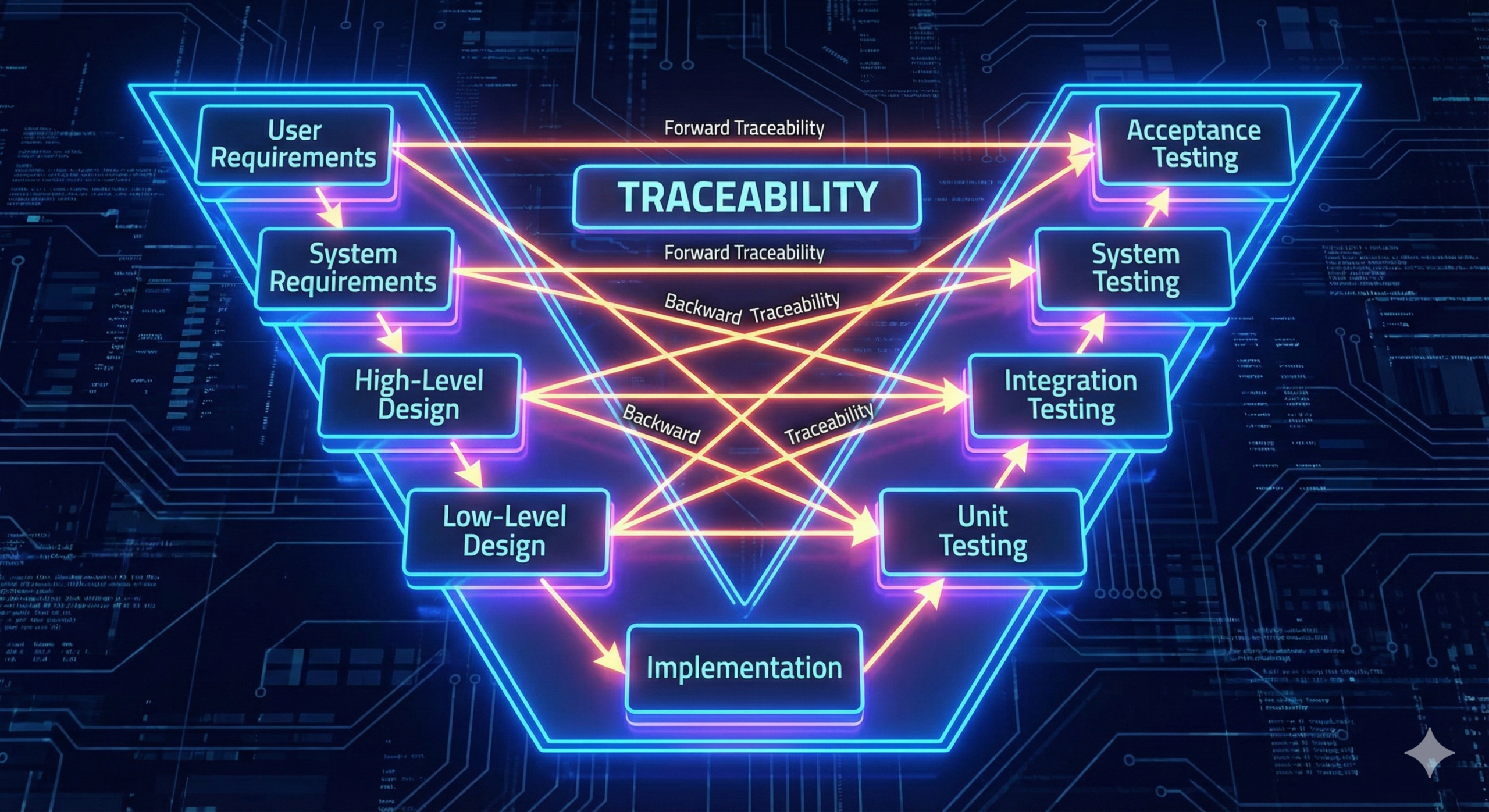
The V-model is a diagrammatic representation of the software system development process, typically expressed as a V-shaped figure. The left side of the V, descending from top to bottom, represents the progressive design process from requirements definition to detailed design. The right side of the V, ascending from bottom to top, represents the progressive test and verification process, starting from unit testing and progressing through integration testing, system testing, to conformance testing. This V-shaped structure embodies the fundamental nature of software development, wherein as the design becomes more detailed, the corresponding test phases also begin at lower design levels and progress upward toward higher-level requirement verification.
The V-model is recognized as a recommended development process model in IEC 62304, the international standard that governs software development for medical devices. In particular, from the initial system risk analysis through the progressive phases of requirements definition, design, implementation, verification, and validation, the assurance of traceability is positioned as an indispensable element throughout each stage.
The Significance of Traceability
Traceability extends beyond its literal meaning of “the ability to trace a path or lineage.” In the context of medical device development, traceability refers to a systematic mechanism that ensures requirements defined in the initial stages are fully implemented and thoroughly verified as they progress through the design phase, implementation phase, and test/verification phases.
Specifically, traceability entails establishing clear correspondence between which requirements are implemented in which design documents, which test cases verify those requirements, and how the verification results are recorded. In this manner, transparency and accountability are secured throughout the entire development process. This serves an essential role in demonstrating to regulatory authorities (FDA, PMDA, EMA, etc.) the evidence underpinning the safety and effectiveness of a medical device in submission dossiers.
IEC 62304 requires the establishment of traceability between various documents: software requirements specifications, software design specifications, software unit design documents, software test plans, test specifications, and test result reports. Similarly, the EU MDR (Medical Device Regulation) and IVDR (In Vitro Diagnostic Regulation) require the submission of traceability evidence covering requirements, design, and testing as part of the technical documentation.
Utilization of the Traceability Matrix
The practical tool for implementing traceability is the “traceability matrix.” This is a table that systematically organizes the correspondence between requirements, design, implementation, and test cases. It serves as an extraordinarily powerful tool for conducting efficient quality assurance activities and fulfilling accountability obligations to regulatory authorities.
A traceability matrix typically includes the following elements:
| Element | Description |
| Requirement ID | A unique identifier assigned to each requirement |
| Requirement Description | A clear statement of the requirement’s content and purpose |
| Corresponding Design | References to the design documents or sections that implement the requirement |
| Corresponding Test Cases | References to the test cases that verify the requirement |
| Verification Status | A status indicator showing whether verification is complete |
| Remarks | Supplementary explanations regarding the correspondence |
For example, consider participation in a large-scale medical device development project. Dozens or even hundreds of requirements may be identified, making it challenging to comprehend and manage them all. With a traceability matrix in place, one can immediately confirm that requirement “REQ-001: The device shall have the capability to automatically save patient data” is implemented in design document “SFD-01,” section “3.2,” and verified through test cases “TC-045” and “TC-046.” Furthermore, should a requirement change occur, the traceability matrix enables rapid identification of which portions of the design and testing would be affected, facilitating efficient impact analysis.
Traceability and Regulatory Requirements
The regulatory landscape for medical devices has undergone significant evolution in recent years. The FDA (U.S. Food and Drug Administration) revised its “Software Validation Guidance” in 2023, further emphasizing the importance of traceability in software development processes. Particularly for software in medical devices employing artificial intelligence (AI), traceability extending from training datasets through design, testing, and clinical verification has become a critical consideration in compliance assessment.
The EU MDR and IVDR explicitly require the submission of traceability evidence regarding requirements, design, and testing as part of quality management system documentation in technical documentation annexes II and III. The latest guidance from the European Commission’s Medical Device Coordination Group (MDCG) in 2024, such as MDCG 2024-4 “Software as a Medical Device (SaMD) – Guidelines on Qualification and Classification,” repeatedly emphasizes the importance of traceability evidence as a basis for demonstrating software quality and safety.
The PMDA (Pharmaceuticals and Medical Devices Agency) of Japan also requires, in its medical device review guidance, the establishment of traceability from requirements through implementation and verification for software-related components. This traceability serves as the foundation for evaluating the reliability and safety of medical device software.
Practical Points for Implementing Traceability
To effectively implement traceability, several practical considerations are essential. First, the mechanism for managing traceability should be incorporated into the project design from the earliest stages. Attempting to create a traceability matrix retroactively when development is already underway risks substantial rework and delays.
Second, unique identifiers should be assigned to all artifacts—requirements, design documents, test cases, and so forth. This enables mechanical tracking of correspondences and minimizes the risk of omissions or errors.
Third, the traceability matrix should not be viewed merely as a documentation task, but rather as a quality management tool for the entire development process. When design changes or test failures occur, reference to the traceability matrix enables rapid identification of impact scope and facilitates planning of necessary follow-up activities.
Fourth, the use of tools to manage traceability is highly effective. Implementation can range from spreadsheets (Excel, Google Sheets) for smaller projects to Application Lifecycle Management (ALM) tools or quality management system (QMS) platforms with integrated traceability management capabilities for larger initiatives. These tools provide automated cross-reference checking, integration with change management, automatic audit trail recording, and other functions that substantially reduce the burden of manual management.
Organizational Change and the Role of Traceability
When organizational restructuring or changes in development team composition occur, transferring project history and progress status to new team members becomes a critical challenge. With a traceability matrix and the detailed documentation it supports, new team members can rapidly acquire a comprehensive understanding of the full scope from initial requirements through current design and test status.
Additionally, when faced with changes in the regulatory environment—such as expansion to new markets or adaptation to new regulatory requirements—a traceability matrix enables efficient analysis of which portions of existing development artifacts already address new requirements and which areas require additional work.
Conclusion
The V-model and traceability form the foundation of quality and safety assurance in medical device software development and the building of trust with regulatory authorities. While these concepts may appear abstract, their true value becomes evident only when learned through concrete examples and applied in real development environments.
Particularly for organizations engaged in medical device development, the establishment and maintenance of traceability mechanisms represent far more than a regulatory compliance exercise. Rather, they constitute a strategically important undertaking that enhances the transparency and efficiency of development processes and fundamentally assures product quality and safety. We encourage you to engage with the implementation of the V-model and traceability in your own work environments when the opportunity arises.

Comment