Why do we misstep on the gas pedal and brake?
Recently, we have seen many reports of accidents caused by mistakenly stepping on the gas pedal and brake.
Many readers may wonder why they mistakenly step on the accelerator and brake.
Many causes have been cited, but one of them may be the occurrence of distracting events while driving.
When a cell phone call or other call is received while driving, the driver’s attention may be distracted and he or she may misplace the accelerator and brake.
In addition, if an unexpected event suddenly occurs, such as a person or animal jumping out of the car, and the driver has to step on the brake, his/her judgment may be impaired and he/she may mistakenly step on the gas pedal and brake.
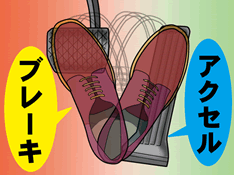
The reason why people misstep on the gas pedal and brake pedal when their attention span or judgment is impaired is that people tend to choose the function they use more often in their daily lives (i.e., the function they are accustomed to).
If one were to ask whether one presses more on the gas pedal or the brake while driving, it is, of course, the gas pedal.
Thus, when attention and judgment are impaired, they unconsciously step on the accelerator, to which they are more accustomed.
Since the person thinks he or she is stepping on the brake, he or she will step on the accelerator with full force.
This causes unexpected acceleration and the driver panics.
The same is true in the design of medical devices. For example, if the operability or interface is different from that of similar or prior products (e.g., reversing red and blue), users may unconsciously operate the device in the way they are used to, which may induce accidents.
As another example, an airplane pilot cannot pilot more than one aircraft at a time. The captain of a Boeing 787 cannot pilot a Boeing 777 at the same time.
Safety design (e.g., fail-safe design) based on human factor engineering (HFE) is important for safety-critical products such as medical devices.
Important for Usability Engineering of Medical Devices
Usability includes “ease of use,” “user satisfaction,” and “appearance,” but what is required in medical device design and development is a “safe” medical device that is error-free in use.
Therefore, usability engineering applied to medical devices is not an evaluation of ease of use, but rather an analytical method for designing safe medical devices free of usage errors.
Making it difficult to use on purpose is also usability.
For example, disposable lighters have heavier knocks. The reason for this is to prevent unexpected ignition by accident (e.g., unintentional or childish mischief).
FDA has historically used the term human factors engineering because the term usability conjures up images of ease of use.
However, because of confusion with international standards, the 2016 publication of the Applying Human Factors and usability Engineering to Optimize Medical Device Design” in defining the terms usability and The definition of the terms in “_blank”>Applying Human Factors and usability Engineering to Optimize Medical Device Design acknowledges that usability and human factors engineering are synonymous.
Revision of JIS T62366-1
Currently, IEC-62366-1:2015 “Medical Devices – Part 1: Application of Usability Engineering to Medical Devices” is a JIS but not yet a basic requirement standard for medical device applications.
However, on July 27, 2021, the Ministry of Health, Labour and Welfare (MHLW) released a draft revision of “JIS T62366-1 Medical devices – Part 1: Application of usability engineering to medical devices” Call for Public Comments. …
This revision is intended to harmonize with the cited standard, JIS T14971:2020.
The main revisions are as follows
- In the “Plan for comprehensive evaluation” in “Establishment of user interface evaluation plan”, for usability testing, the test environment and conditions of use shall be “intended use environment” rather than “actual use conditions” as specified in the current standard, and provisions shall be added to clarify “correct use” for hazard-related use scenarios, considering the characteristics of participants and number of participants in the testing. In consideration of the characteristics of the test participants and the number of participants, a provision has been added to clarify “correct use” for hazard-related use scenarios.
- In addition to “USE ERROR” in the current standard, “USE DIFFICULTY” is added as an observation target in the test.
- In “Conducting a comprehensive evaluation of the usability of the user interface,” analysis of “use difficulty” was added to the current standard’s “use error” as a target of analysis in the comprehensive evaluation.
Perhaps after the revision, compliance with JIS T62366-1 will become the basic requirement standard.
For more information, see here.
related product
[blogcard url=https://xn--2lwu4a.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ]]]>

Thanks in support of sharing such a good thinking, article is nice,
thats why i have read it entirely