Competence Table and Training Needs
The FDA requires that competence management be implemented for personnel engaged in processes that affect product quality.
ISO 9000:2015, Quality Management Systems – Basics and Terminology, defines competence as follows
3.10.4 competence
The ability to apply knowledge and skills to achieve intended results. In other words, competence is not only about knowledge, but also about skills.
Skills can be acquired through training. In many companies, group training is regulated, but training (on-the-job training) is not, or if it is regulated, there are no records of it.
Training Needs
What is important here is the identification of training needs.
In other words, they determine who in the department should have what competencies and impose the necessary training accordingly.
It is not necessary for all personnel in a department to have the same competencies; it is important to achieve a balance within the department for each competency. For this purpose, it is necessary to create a competence table for each department.
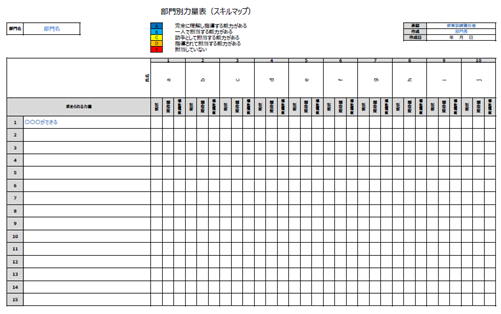
Departmental Strength Scale
A color-coded display of the current competence of all personnel in the department would give a glimpse of the department’s weak points.
Therefore, consider imposing training on certain personnel.
In the individual competence table for the personnel in question, a target value for the competence in question will be set. In addition, it is to be determined whether the training has resulted in the expected competence at the end of the half-year or the fiscal year.
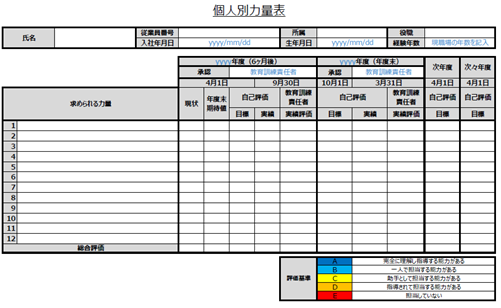
Individual Competency Tables Thus, competency tables, both departmental and individual, are needed and used to identify training needs.


Comment