Overview
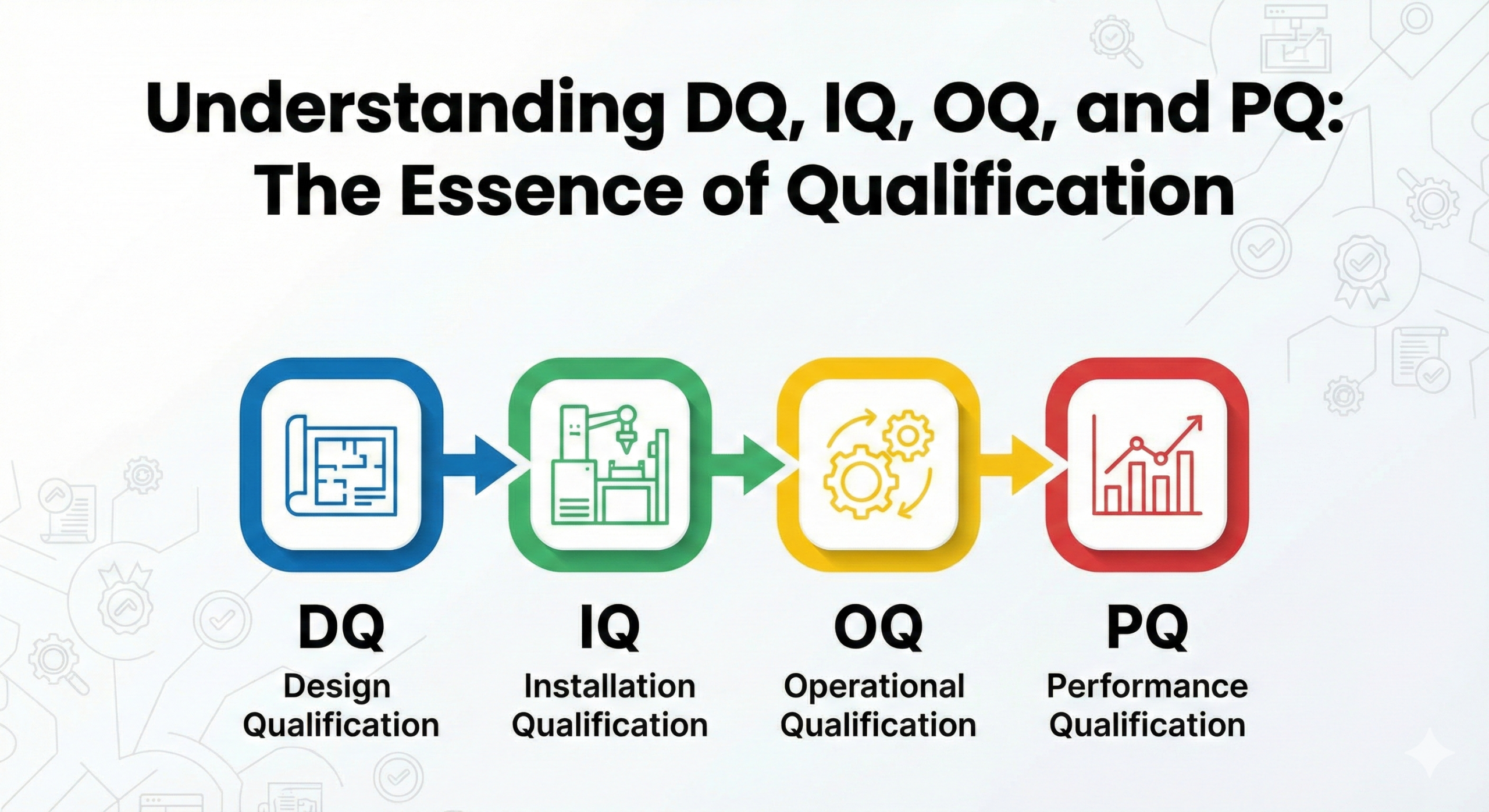
DQ, IQ, OQ, and PQ are fundamental qualification methods used in the validation of structural equipment and facilities within the pharmaceutical and biotechnology industries. These terms refer to Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Originally developed as concepts within process validation, they are now widely applied in the field of Computer System Validation (CSV).
This phased approach is a systematic method for confirming that hardware and systems are “qualified” to meet user requirements. It is a regulatory requirement mandated by Japan’s Ministry of Health, Labour and Welfare (MHLW) Validation Guidance (issued in April 2021, enforced in August 2021) and the PIC/S GMP Guidelines. In Japan, validation standards were first issued in August 2013 (Heisei 25), and subsequently, with the enforcement of the revised GMP ministerial ordinance in 2021 (Reiwa 3), the nomenclature was changed from “Validation Standards” to “Validation Guidance.” This revision was implemented as part of international harmonization efforts following Japan’s accession to PIC/S.
Detailed Description of Each Stage
DQ (Design Qualification)
Definition: The documented verification that the proposed design of equipment, facilities, or systems is suitable for the intended purpose.
In DQ, documentary review is conducted to confirm that the design specifications of systems and equipment meet the User Requirements Specification (URS) and regulatory requirements. By evaluating qualification at the design stage before actual equipment is installed, risks in subsequent processes can be minimized.
Importance: Insufficient attention to DQ increases the burden on IQ, OQ, and PQ and elevates equipment risks, making thorough verification at this stage critically important. In PIC/S Annex 15, the URS is positioned as a crucial document that should be referenced throughout the validation lifecycle, and the application of a quality risk management approach is required.
IQ (Installation Qualification)
Definition: The documented verification that equipment or systems, as installed or modified, comply with approved design and manufacturer’s requirements.
IQ involves actual inspection to verify that equipment is correctly installed and connected according to design specifications. This includes detailed confirmation of piping, electrical wiring, instrument installation status, and verification of consistency with approved drawings and specifications. Specifically, this encompasses confirmation of materials of construction, suitability of installation environment, verification of utility connections, and confirmation of calibration certificates.
OQ (Operational Qualification)
Definition: The documented verification that equipment or systems, as installed or modified, perform as intended throughout the anticipated operating ranges.
OQ is the stage where testing confirms that equipment functions as intended within its designed operating range. This systematically includes operational confirmation at various parameter settings, verification of control functions, confirmation of alarm function operation, and operational confirmation under worst-case conditions. At this stage, it is important to objectively demonstrate that equipment operating parameters meet specifications using appropriately calibrated measuring instruments.
PQ (Performance Qualification)
Definition: The documented verification that equipment and ancillary systems are capable of consistently operating in accordance with approved manufacturing methods and product specifications effectively and reproducibly.
PQ demonstrates that equipment can continuously produce products meeting specifications under actual manufacturing conditions. By collecting performance data in actual production environments and confirming conformity to quality standards and reproducibility, transition to commercial production becomes possible. PQ is normally performed following successful completion of IQ and OQ, but in some cases may be conducted concurrently with OQ or Process Validation.
Phased Implementation of Qualification
| Stage | Primary Purpose | Implementation Timing | Main Verification Items |
|---|---|---|---|
| DQ | Design suitability confirmation | Before equipment installation | URS conformity, regulatory compliance, risk assessment |
| IQ | Installation accuracy confirmation | After equipment installation | Materials of construction, piping/wiring, environmental conditions, calibration certificates |
| OQ | Operational function confirmation | During equipment commissioning | Operating range, control functions, alarms, worst-case scenarios |
| PQ | Manufacturing performance demonstration | Before operational start | Product quality, reproducibility, continued performance |
Application in CSV
Computer System Validation (CSV) is defined as “the process of confirming that regulated computerized systems operate in a consistent and reproducible manner according to design, with the same safety, reliability, and dependability as paper-based records.”
For integrated systems combining software and hardware, DQ, IQ, OQ, and PQ methods are effectively utilized, enabling phased demonstration of system reliability and regulatory compliance. PIC/S Annex 15 explicitly states that details regarding computerized systems should reference Annex 11 separately, requiring distinction between application validation and IT infrastructure qualification.
Furthermore, in July 2025, the EU and PIC/S published draft revisions to Annex 11 (Computerised Systems) and Annex 22 (Artificial Intelligence) for public comment, advancing regulatory development to address progress in digital and AI technologies.
Benefits of the Phased Approach
This systematic four-stage approach realizes the following benefits:
Risk Minimization: Early-stage problem identification avoids major modifications in later processes. Application of quality risk management approaches (ICH Q9) enables efficient, scientifically-based validation.
Regulatory Compliance: Systematic demonstration in accordance with domestic and international regulatory guidelines is possible. Japan’s Validation Guidance is harmonized with PIC/S Annex 15, enabling internationally acceptable quality assurance.
Quality Assurance: Scientific evidence can be established to ensure patient safety and product quality. Within the framework of the Pharmaceutical Quality System (ICH Q10), continuous improvement and product lifecycle management are supported.
Efficient Business Operations: Planned verification improves project predictability. By developing a Validation Master Plan (VMP), organizational validation activities can be systematically managed.
Latest Regulatory Trends
The revised GMP ministerial ordinance enforced in August 2021 (Reiwa 3) significantly strengthened requirements related to validation. Major changes include explicit requirements for establishing quality risk management and mandatory establishment of quality assurance and testing departments under the quality department. Additionally, preparation of the Validation Master Plan was clarified, and the concept of Continued Process Verification (CPV) was introduced.
Furthermore, Retrospective Validation is no longer internationally accepted and is not recognized as an acceptable approach in PIC/S Annex 15 (enforced in 2015). Currently, prospective validation, concurrent validation, and hybrid approaches are positioned as standard methods.
Conclusion
DQ, IQ, OQ, and PQ are not merely procedural requirements but represent a scientific and practical approach to ensuring product quality and patient safety. In pharmaceutical manufacturing settings, proper understanding and application of these concepts constitute an essential methodology that enables both regulatory compliance and efficient business operations.
With the 2021 revision of GMP ministerial ordinance and transition to Validation Guidance, international harmonization has further advanced, requiring systematic validation implementation based on quality risk management and lifecycle approaches. By properly understanding these requirements and implementing them organization-wide, pharmaceutical companies can provide safer, higher-quality medicines to patients.

Comment