What is good quality?
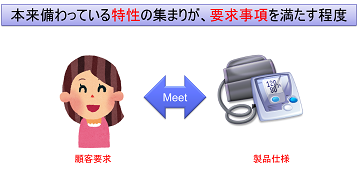
ISO-9000 defines quality as “the degree to which a collection of inherent characteristics satisfy requirements” is defined as “the degree to which a collection of inherent characteristics satisfies the requirements </
A state in which the customer’s requirements and product specifications match, which is called a state of good quality.
Good or bad usability is not inherently related to quality.
Let us assume, for example, that there was no scalpel when performing surgery.(unlikely, but…)
A quality, top-quality knife was purchased to replace the scalpel.
However, even the finest knives cannot be used for surgery.
This is because they are not suitable for surgery.
If the specifications of the medical device are not met for the intended use (Intended use), accidents will occur.
In other words, the finest knives are not good quality medical devices.

Let me illustrate with another example.
Suppose you want to tighten a Phillips screw at home, but you don’t have a Phillips screwdriver.
Phillips-head screws can also be tightened with a flat-blade screwdriver.
However, tightening Phillips-head screws with a flat-blade screwdriver may crush the screw holes.
If repeated, the driver itself could be damaged.
In short, a flathead screwdriver is not compatible with the intended use of tightening Phillips screws.

Fitness for purpose
The medical device regulations do not regulate only the quality of the medical device itself. The “has-inline-color has-vivid-red-color” class=”has-inline-color has-vivid-red-color”>is not solely regulating the quality of the medical device itself.
It is important that the specifications of the relevant equipment match the actual medical practice, patient, and user usage.
This is called fitness for purpose (fitness for purpose).
If the specifications of the relevant medical device do not match the intended use, accidents will occur.
In this case, it means that the medical device is not of good quality.
related product
[blogcard url=https://xn--2lwu4a.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/QMS-MHLW-01.html title=”【2021年度改正QMS省令対応】品質管理監督システム基準書”] [blogcard url= https://ecompliance.co.jp/SHOP/QMS-MHLW-05.html title=”【2021年度改正QMS省令対応】設計管理規程・手順書・様式”] [blogcard url= https://ecompliance.co.jp/SHOP/O007.html title=”【VOD】医療機器の薬機法入門セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-114.html title=”【セミナービデオ】QMSRセミナー (Quality Management System Regulation)”]]]>


Comment