…
What is the Basic Requirements Criteria?
What are the approval standards for medical devices?
Approval criteria” means criteria for medical devices for which approval review is conducted by confirming conformity to such criteria.
The “Approval Criteria” are, in principle, composed of international standards, etc., and specify the scope of items that do not need to be accompanied by data on clinical trial results.
Contents of Approval Criteria
1.scope (of a document)
Designate the subject medical device by its generic name
2.technical standards
Items related to performance, function, effectiveness, etc.
3.Purpose of Use, Indications or Effects
Limit the intended use, indications or effects subject to the criteria
4.Conformance to basic requirements
Explanation of conformance to basic requirements
5.Other
If the structure, method of use, performance, etc. are clearly different from those of existing medical devices, the device shall not conform to the criteria.
The fourth of the approval criteria for medical devices is conformity to the Basic Requirements Criteria. The certification criteria for medical devices also require conformity to the basic requirement criteria in the same manner.
What is the Basic Requirements Criteria?
The Basic Requirements Standard specifies the basic requirements for quality, efficacy, and safety that all medical devices or in vitro diagnostic products must possess.
When applying for marketing authorization/certification or notification of manufacturing and marketing of a medical device, materials regarding conformity with the basic requirement criteria must be submitted.
Basic requirement standards for medical devices:
“Medicine, Medical device standards specified by the Minister of Health, Labour and Welfare pursuant to the provisions of Article 41, paragraph 3 of the Act on Quality, Efficacy and Safety Assurance of Medical Devices, etc. ” (Ministry of Health, Labour and Welfare Notification No. 122 of 2005)
Basic Requirements Standard for In Vitro Diagnostic Products:
“Medicine, ” (Ministry of Health, Labour and Welfare Notification No. 126 of 2005)
In 2005, the Global Harmonization Task Force (GHTF) defined “common requirements for the design and manufacturing of all medical devices and in vitro diagnostic products to ensure their safety” (i.e., basic requirements). (GHTF/SG1/N). (GHTF/SG1/N41R9:2005)
These basic requirements were revised in 2012 and published as a final document (GHTF/SG1/N68:2012).
Subsequently, in 2018, the IMDRF (International Medical Device Regulators Forum/International Medical Device Regulators Forum) document “Essential Principles of Safety and Performance of Medical Devices and IVD Medical Devices” (IMDRF/GRRP WG/N47 FINAL:2018).
In Japan, based on international harmonization, the “Basic Requirements Standards” (Ministry of Health, Labor and Welfare Notification No. 122 of 2005) were established in 2005.
In August 2021, the Basic Requirements Standards were revised in accordance with the revision of the Pharmaceutical Affairs Act.
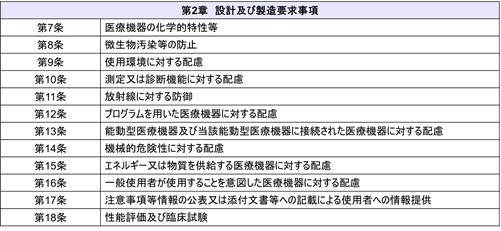
In addition, the “Checklist of Conformity to Basic Requirements for Medical Devices” has been revised accordingly. The Basic Requirements Standard consists of “Chapter 1 General Requirements” and “Chapter 2 Design and Manufacturing Requirements.

As it stands, Article 2, “Risk Management,” states that “Cybersecurity” is interpreted to include “Cybersecurity”. However, the Basic Requirements Standard is scheduled to be revised in the summer of 2023 to clarify cybersecurity requirements, and it is expected that the Basic Requirements Standard will include the following statement: “The system shall be designed and manufactured so that it can demonstrate reproducibility, reliability, and performance throughout the product life cycle. The basic requirement standard is expected to include the following statement
What is the Basic Requirements Conformance Checklist?
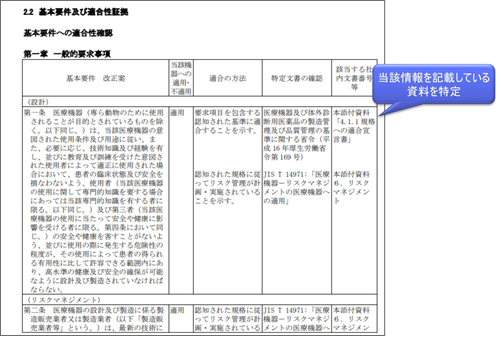
When applying for a medical device, a column titled “Applicable Internal Document Numbers, etc.” will be added to the Basic Requirements Compliance Checklist to identify the documents that contain such information. For details, see “Example of description of materials attached to an application for marketing authorization of a medical device program“.
Medical Device Certification Criteria and Basic Requirements Criteria
For certified products, checklists are individually defined to confirm compliance with the basic requirement criteria.
The contents described in “application/non-application to the device in question,” “method of conformity,” and “confirmation of specific documents” may be changed if there is a scientifically valid reason for doing so. However, if there is any change in “Applicability/non-applicability to the relevant device,” the “intended use or effect” or “definition of generic name” of the device may be deviated from, so prior inquiry to the registered certification body is required.
related product

]]>
Related Articles
- Amendment to the Medical Device Basic Requirements Standard
- The Relationship Between URS (User Requirements Specification) and PQ (Performance Qualification)
- Understanding Data Retention Requirements in Pharmaceutical Regulations
- What is the Electronic Document Law?
- Challenges in Electronic Record Retention Requirements: Maintaining Authenticity During Long-term Preservation and System Migration
- What is Validation (Revised Edition)


Comment