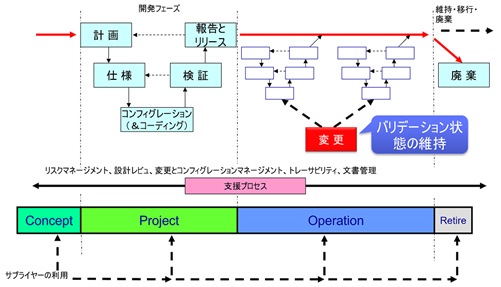
What is maintaining validation status?
In computerized system validation (CSV), the important thing in the operational phase is to maintain the validation status.
To begin with, validation of a computerized system means “matching the specifications of the system to the requirements of the user.
During the project phase, the supplier defines the requirements, specifies, builds, and tests the system to meet user requirements.
In other words, at the end of the project phase, the computerized system specifications meet the user’s requirements.
However, user requirements may change during the operational phase after release (start of operation).
In general, cases where user requirements change are,
- Changes in regulatory requirements
- Changes in interpretation of regulatory requirements
- Organizational Changes
- Product Change
- Change of procedure
Possible examples include.
“2. Changes in the interpretation of regulatory requirements” refers to cases in which regulatory requirements have not been revised, but procedures manuals, etc. are revised when a QMS, etc. is pointed out during inspections or internal audits by regulatory authorities.
The risk may also change due to “4. product changes”.
In general, validation of CSVs and other validations should be A risk-based approach should be used to define the criteria for implementation.
If the product is changed, the risk may change, so it is necessary to re-determine whether the previous level of validation is sufficient.
Thus, during the operational phase, change control must be implemented to ensure that the system specifications always meet user requirements. This is called “maintaining the validation status.
Related products
[blogcard url=https://ecompliance.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ] [blogcard url=https://ecompliance.jp/qms-rx/ title=”QMS(手順書)ひな形 医薬品関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/CSV-5KY-LIVE-000010.html title=”【VOD】CSVセミナー【第10講】”] [blogcard url= https://ecompliance.co.jp/SHOP/L_MDCSV.html title=”【VOD】医療機器企業におけるCSV実践セミナー“] [blogcard url= https://ecompliance.co.jp/SHOP/BOOK-CSV2.html title=”【書籍】【超入門】コンピュータ化システムバリデーション”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-026.html title=”【セミナービデオ】現場目線で考える!すぐ活用出来る! コンピュータ化システムバリデーション(CSV)超入門2020″]


Comment