Common Misconceptions About CAPA
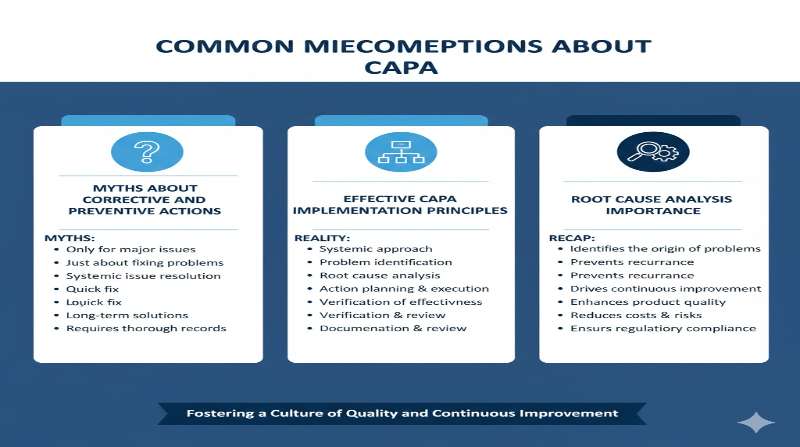
Introduction
Both the QMS Ministerial Ordinance (Ministerial Ordinance on Standards for Manufacturing Control and Quality Control for Medical Devices and In Vitro Diagnostic Reagents) and the revised GMP Ministerial Ordinance (Ministerial Ordinance on Standards for Manufacturing Control and Quality Control for Drugs and Quasi-drugs) naturally require thorough implementation and management of CAPA. However, many companies do not properly understand CAPA.
CAPA stands for Corrective and Preventive Action, and it is a critical element at the core of a Quality Management System (QMS). Regulatory requirements such as the U.S. FDA (Food and Drug Administration) 21 CFR Part 820.100 and international standard ISO 13485:2016 explicitly require proper implementation of CAPA.
About Improvement
The term “Kaizen” is one of the famous Japanese words recognized worldwide.
In the 1970s, Japanese automobiles achieved great success in the U.S. market. Japanese cars were competitively priced while being highly reliable with few breakdowns. They also offered superior fuel efficiency. Feeling threatened, America’s Big Three automakers conducted thorough investigations into the quality control practices of Japanese automobile manufacturers. As a result, they focused their attention on “Toyota’s improvement methods.”
After World War II, Toyota received guidance from U.S. quality control experts Dr. W. Edwards Deming and Dr. Joseph Juran, and introduced Statistical Quality Control (SQC) in 1949. Toyota won the Deming Prize in 1965 and the Japan Quality Control Award in 1970. Toyota’s Kaizen is a core concept of the Toyota Production System (TPS) and has evolved as a continuous improvement activity involving all employees.
This “Kaizen” crossed the ocean to the United States and was customized in the American style. In Japan, QC (Quality Control) activities are often implemented in a bottom-up manner, with shop floor workers taking the lead in small group activities. In contrast, Western countries typically adopt a top-down approach. CAPA can be said to have customized the Japanese “Kaizen” concept into a top-down approach and incorporated it as part of a more systematic quality management system.
In other words, CAPA is Japanese-born but American-raised. However, the current reality is that companies frequently receive citations from FDA inspections and other regulatory audits regarding CAPA implementation and management. This is despite continuous improvement originally being a Japanese specialty.
In fact, inadequate CAPA is one of the most common observations in FDA 483 warning letters. It is said that the FDA has harshly evaluated that “Japanese companies do not understand CAPA at all.”
The Difference Between Correction and Corrective Action
Regulatory requirements and international standards (ISO 9001, ISO 13485, etc.) clearly distinguish between Correction and Corrective Action.
Correction is an action to eliminate a detected nonconformity and addresses the immediate cause. In other words, it refers to the act of immediately resolving the problem itself that has occurred.
On the other hand, Corrective Action is an action to eliminate the cause of a detected nonconformity or other undesirable situation, addressing the root cause. Its primary purpose is to prevent recurrence.
In ISO 9000:2015 and ISO 13485:2016, these definitions are clarified as follows:
- Correction: Action to eliminate a detected nonconformity
- Corrective Action: Action to eliminate the cause of a nonconformity and prevent recurrence
Explanation Through a Specific Example
For example, suppose there was an accident where a pipe in a piece of equipment cracked and water leaked.
Examples of Corrections:
- “Wrap tape around the pipe”
- “Replace the pipe”
- “Wipe up the leaked water”
These are actions that resolve the immediate problem.
However, if you end with just corrections, the same problem may recur.
Therefore, you must investigate the root cause of why the pipe cracked. Root cause analysis methods include “5 Whys Analysis,” “Cause and Effect Diagram (Fishbone Diagram),” and “FTA (Fault Tree Analysis).”
Examples of Root Causes:
- “The pipe material was not suitable for the operating environment”
- “There was a problem with the pipe installation angle, causing excessive stress concentration”
- “The pipe was used at pressures or temperatures exceeding assumptions”
- “Regular inspection and maintenance were not performed”
Examples of Corrective Actions:
- “Review pipe material specifications and select appropriate materials”
- “Revise design standards and standardize installation methods that avoid stress concentration”
- “Establish procedures for managing operating conditions and strengthen monitoring of operating parameters”
- “Develop a periodic inspection program and implement preventive maintenance”
If you do not identify and resolve the root cause in this way, the problem will recur.
A Common Misconception: Design Changes Are Not Corrective Actions
In my consulting work, I often encounter companies that, when a design error occurs, only implement a design change (drawing revision) as their corrective action.
However, a design change (drawing revision) is a correction, not a corrective action.
The reason is that a design change (drawing revision) alone does not prevent similar problems from recurring in other areas. A design change directly corrects the problem itself but does not address the root cause.
As a corrective action, you need to investigate the root cause of “why such a design error occurred” and implement system-level recurrence prevention measures.
Examples of Proper Corrective Actions:
- Revise design review checklists
- Strengthen design verification and validation procedures
- Review training programs for designers and improve design standard documents
- Improve risk management processes to enable early identification of similar risks
Training Is Not a Corrective Action
Another common example of incorrect CAPA that I frequently encounter is determining the root cause as “insufficient training” and the corrective action as “thorough retraining.”
However, this is insufficient as a corrective action. When training is provided, the trainees may deepen their understanding and temporarily avoid making the same mistake. However, if personnel change due to organizational restructuring or if memory fades over time, the same mistake may occur again.
In other words, training alone is insufficient for preventing recurrence, and it is unacceptable to use only measures such as “thorough training” as a corrective action.
Appropriate Approach to Corrective Action
Even when training is deemed necessary, it is only part of the corrective action. True corrective action involves modifying the QMS (Quality Management System) itself—the mechanism or system. It is essential to implement permanent measures that prevent the same errors from occurring even when personnel change.
Examples of System-Level Corrective Actions:
- Revise work procedures to create processes less prone to errors
- Introduce checklists to establish mechanisms that don’t overlook critical steps
- Incorporate Poka-Yoke (error-proofing) mechanisms into the design
- Establish a double-check system and standardize mutual verification processes
- Introduce automated checking functions through electronic systems
- Systematize training programs and establish competency assessment mechanisms (improving the training system itself, not just providing training)
FDA and ISO 13485 require verification of CAPA effectiveness. Therefore, it is essential to confirm and document whether the implemented corrective actions were actually effective in preventing recurrence.
Summary
To properly understand and implement CAPA, you need to grasp the following key points:
- Clearly Distinguish Between Correction and Corrective Action
- Correction addresses the immediate cause (fixes the problem itself)
- Corrective action addresses the root cause (prevents recurrence)
- Thoroughly Conduct Root Cause Analysis
- Don’t be satisfied with superficial causes; identify the true root cause
- Appropriately utilize methods such as “5 Whys Analysis”
- Implement System-Level Improvements
- Build mechanisms that don’t depend on individual capability or attention
- Improve the QMS itself and implement permanent recurrence prevention measures
- Verify the Effectiveness of Corrective Actions
- Confirm whether implemented corrective actions were actually effective
- Continuously monitor and implement additional measures as necessary
CAPA is not merely a documentation exercise but an important tool for achieving organizational continuous improvement and quality enhancement. Properly practicing the spirit of Japanese-origin “Kaizen” in a form that complies with modern regulatory requirements is the cornerstone of quality assurance in the pharmaceutical and medical device industries.
References and Regulatory Requirements:
- FDA 21 CFR Part 820.100 – Corrective and Preventive Action
- ISO 13485:2016 – Medical devices — Quality management systems
- ISO 9001:2015 – Quality management systems — Requirements
- ISO 9000:2015 – Quality management systems — Fundamentals and vocabulary
- ICH Q10 – Pharmaceutical Quality System
Related Articles
- Understanding Quality Systems in Pharmaceutical and Medical Device Industries
- Why ISO-9001 Certification Alone Does Not Guarantee Quality Improvement
- The Critical Importance of Root Cause Analysis
- The Importance of Root Cause Analysis in CAPA
- The Declining Quality Culture in Japan: A Critical Analysis with Current Regulatory Perspectives
Related FDA QMSR Templates
Streamline your FDA QMSR compliance with our professionally crafted templates:


Comment