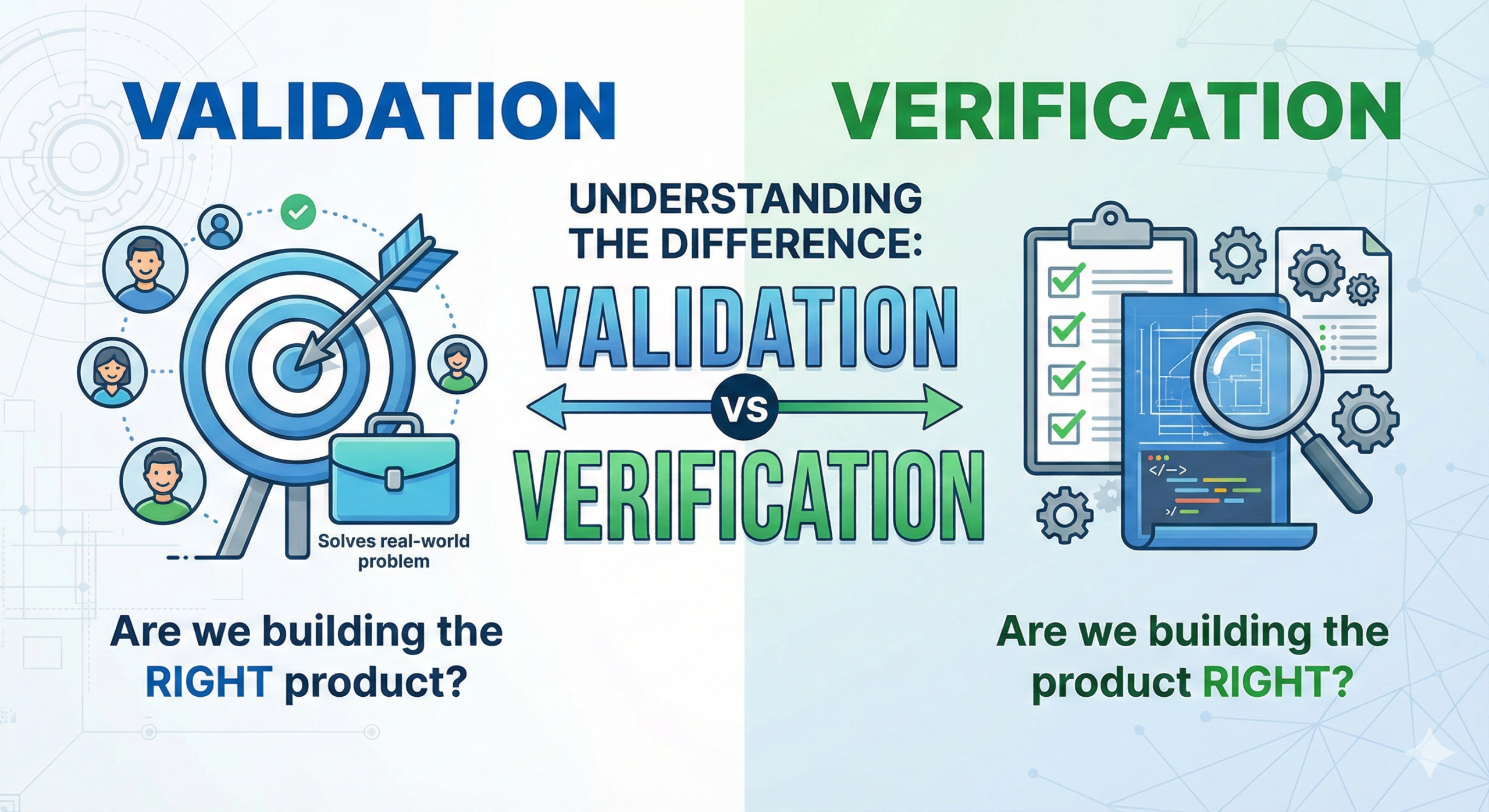
Understanding the Difference Between Validation and Verification
The author frequently receives questions about the difference between validation and verification.
The FDA treats “verification” and “validation” as separate and distinct terms. On the other hand, many industries use the terms “verification” and “validation” interchangeably, or refer to them as a single concept such as “V&V,” considering that no distinction exists.
So, what is the difference between validation and verification as defined by the FDA?
Definition of Validation
Validation is:
Demonstrating that products conforming to design quality can be consistently manufactured in the future. It is proving that processes can be controlled according to established parameters and that products consistently meeting specifications can be manufactured.
Definition of Verification
Verification is:
Confirming that a product conforming to design quality has been manufactured. It is confirming that a process has been controlled according to established parameters and that a product meeting specifications has been manufactured.
A Simple Way to Remember the Difference
In other words, “validation” is future-oriented, while “verification” is past-oriented. Remembering it this way makes it easier to understand.
However, regardless of the approach, the ultimate purpose of both is patient protection. In other words, the objectives of validation and verification are ultimately the same: to ensure product quality and secure patient safety. Whether validation is performed in advance or verification is performed afterward, the quality of products to be manufactured or already manufactured must be highly assured, and patient safety must be guaranteed.
When is Validation Necessary?
Validation is necessary for processes that can only be evaluated through destructive testing.
This is because once destructive testing is performed, the product cannot be shipped. Therefore, sampling inspection must be implemented. However, sampling inspection has its limitations. For example, suppose 10,000 potato chips are manufactured. If 30 samples are taken from these and inspected for foreign matter, and no foreign matter is found in any of them, there remains a possibility that foreign matter may be present in any of the remaining 9,970 chips. This is the limitation of sampling inspection.
Therefore, validation must be performed in advance to ensure that foreign matter will absolutely not be introduced.
Application in Different Industries
Process Industries (Pharmaceutical Manufacturing)
Generally, in process industries such as pharmaceutical plants, destructive testing (sampling inspection) is fundamental, so validation must be implemented for almost all processes.
Discrete Manufacturing Industries (Medical Device Manufacturing)
However, in discrete manufacturing industries such as medical device companies, verification is central. This is because most medical devices can be subjected to non-destructive testing. For example, measurement with an oscilloscope, testing with a tester, or X-ray projection. In other words, 100% inspection is possible.
Therefore, manufactured products (including intermediate products) can be verified, and if any non-conforming products (defective products) are found, they can be discarded or reworked (remanufactured). Subsequently, only conforming products can be shipped.
Special Processes in Medical Device Manufacturing
However, even in medical devices, there are processes that involve destructive testing. Examples include sterilization, soldering, crimping, bonding, welding, and compression bonding.
For example, whether bonding has been sufficiently achieved cannot be determined by visual inspection, so destructive testing must be performed. Therefore, sampling testing is unavoidable.
Processes that cannot be sufficiently verified in subsequent processes are called special processes.
For special processes, validation must be performed in advance to highly assure that the process output (intermediate products, finished products, etc.) meets specifications.
Comparison Table
| Aspect | Validation | Verification |
| Temporal Orientation | Future-oriented (prospective) | Past-oriented (retrospective) |
| Definition | Proving that conforming products can be consistently manufactured | Confirming that a conforming product has been manufactured |
| When Applied | Before routine production, for processes that cannot be fully verified | After manufacturing, for processes that can be fully inspected |
| Primary Use | Process industries (pharmaceutical manufacturing), special processes | Discrete manufacturing (medical devices) with non-destructive testing capability |
| Testing Approach | Sampling inspection with high assurance | 100% inspection (when possible) |
| Typical Processes | Sterilization, bonding, welding, crimping, soldering | Electrical testing, dimensional inspection, X-ray inspection |
| Regulatory Emphasis | FDA 21 CFR Part 820, ISO 13485:2016 Clause 7.5.6, EU MDR Annex IX | ISO 13485:2016 Clause 7.5.2, FDA 21 CFR Part 820.75 |
Regulatory and Standards Context
The distinction between validation and verification is formalized in various international standards and regulations:
ISO 13485:2016 (Medical devices – Quality management systems) clearly distinguishes between validation of processes for production and service provision (Clause 7.5.6) and verification activities (Clause 7.5.2). The standard requires organizations to validate processes where the resulting output cannot be verified by subsequent monitoring or measurement, and where deficiencies may become apparent only after the product is in use or the service has been delivered.
FDA 21 CFR Part 820.75 (Process Validation) specifically requires that where the results of a process cannot be fully verified by subsequent inspection and test, the process shall be validated with a high degree of assurance and approved according to established procedures.
EU Medical Device Regulation (MDR 2017/745) reinforces these requirements through Annex I (General Safety and Performance Requirements) and conformity assessment procedures that mandate process validation for critical manufacturing processes.
ISO 9001:2015 also maintains this distinction in Clause 8.5.1, requiring validation of processes where output cannot be verified by subsequent monitoring or measurement.
Conclusion
Understanding the distinction between validation and verification is fundamental to ensuring regulatory compliance and patient safety in both pharmaceutical and medical device manufacturing. While the terminology and emphasis may vary across industries, the core principle remains constant: ensuring that products consistently meet quality specifications and protect patient safety, whether through prospective process validation or retrospective product verification.
The choice between validation and verification depends primarily on whether the process output can be fully verified through non-destructive testing. When destructive testing is required or when process results cannot be fully verified afterward, validation becomes not just a regulatory requirement but a critical quality assurance tool.

Comment