From “Validation” to “Verification
More than 20 years ago, in December 2001, GAMP 4 was published. The title was “GAMP Guide for Validation of Automated Systems.
In fact, GAMP 4 has a history of FDA participation in the review process. Therefore, GAMP 4 became the de facto standard for CSV at that time.
However, there was a major problem. That is, compliance costs.
This was because, at the time of GAMP 4, double quality assurance was being implemented, with the pharmaceutical company repeating the testing and other activities conducted by the supplier.
The compliance costs used by the company are passed directly onto the price of the product, which is ultimately borne by the patient.
ASTM E2500
So the FDA commissioned the American Society for Testing and Materials (ASTM), an organization with no ties to the pharmaceutical industry, to develop guidelines for testing new drug manufacturing equipment.
ASTM develops test methods, standards, and specifications for materials, products, systems, and services. With members in more than 100 countries, test methods and standards developed by ASTM are used by companies and government agencies worldwide to ensure product safety, quality, and performance.
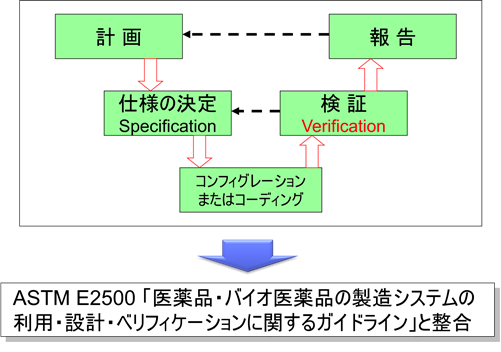
At the request of the FDA, ASTM developed ASTM E2500 “Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment (Guidelines for the Use, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems)” in 2007.
The following year, in 2008, ISPE, which presides over GAMP, published GAMP 5 “A Risk-Based Approach to Compliant GxP Computerized Systems,” which was harmonized with ASTM E2500.
The most significant difference between GAMP 4 and GAMP 5 is the disappearance of the term Validation from the title.
In other words, the guidance was changed to Verification, not Validation, to align with ASTM E2500. This is not well known.
General Specification & Verification Approach
The approach to specification and verification in GAMP 4 (English version) – the so-called V-model – described Validation in the position of verification.
However, in GAMP 5 (English version), verification has been changed to Verification.
However, in GAMP 5 (Japanese version), both remain validation, so the change is unnoticeable.
In GAMP 4, activities conducted by suppliers were repeated again by pharmaceutical companies as Validation. This is a waste of compliance costs.
Under GAMP 5, the supplier’s activities are verified by the pharmaceutical company. This prevents duplication of work between suppliers and pharmaceutical companies.
Many Japanese companies are unaware of this and are still implementing rigid Validation activities. Quality assurance is an important activity, but it should not be excessive.


Comment