What are the conditions for process validation?
ISO 13485 7.5 Manufacturing and service provision 7.5.6 Process validation for manufacturing and service provision contains the following requirements
The outputs resulting from the process of manufacturing and service provision that areimpossible to verify with subsequent monitoring or measurement or that are not verified, or does not perform validation, and the resulting defects only become apparent after the product has been used or services have been provided, the organization performs the applicable process validation of its manufacturing and service provision.
Also, §820.75 Process Validation of the FDA QSR contains the following requirements The following requirements are listed in §820.75 Process Validation of the QSR.
When the results of a process cannot be adequately verified by post-process inspection and testing, they shall be validated with a high degree of confidence and approved according to established procedures.
In other words, the conditions under which process validation must be performed are those that cannot be adequately verified by inspection or other means after manufacture.
The processes (processes) that involve destructive inspections are those that cannot be sufficiently verified. A process that involves destructive testing is called a “special process.
Examples are processes such as soldering, crimping, sterilization, gluing, welding, crimping, etc.
Sterilization must be tested by destroying the sterile bag or other sterile barrier system to determine if sterilization has been properly performed.
However, if they are destroyed, they cannot be shipped as products.
Therefore, special processes involving destructive inspection inevitably result in sampling inspections. In other words, it is not possible to inspect all products.
The problem with sampling inspections is that quality defects may exist in products that were not sampled.
In the unlikely event that a product is shipped with a sterilization defect, it must not be allowed.
Therefore, in special processes, rather than relying on post-manufacturing inspections, a high level of quality assurance must be implemented in advance to ensure that the results of the process in question are “one in a hundred”. For example, if 10,000 products are manufactured, all 10,000 must be completely sterilized.
This is the purpose of process validation.
Many medical devices are capable of nondestructive testing. For example, visual inspection, oscilloscope measurement, tester measurement, and power meter measurement. In such processes, quality can be adequately assured by post-processing inspections. On the other hand, for special processes where sufficient verification is not possible, process validation must be performed in advance.
Once upon a time, the author audited the manufacturing facility of a medical device company. At that time, I noticed something strange about the way an employee who was performing hand soldering was holding the soldering iron. When he returned to his living room and showed me the employee’s education and training record, I found that the test result for soldering was 60 points.
The author noted in the audit report that a person with a score of 60 should not be allowed to perform soldering operations.
However, the audited department responded that whether a score of 60 is a pass or fail is a subjective matter. In other words, the intent was that the criteria would not be mentioned anywhere in the regulatory requirements.
This is not a story at all.
The soldering process is a special process. Subsequent inspections (e.g., visual inspections) cannot adequately verify the process. Therefore, the personnel who perform the soldering process must have a perfect score of 100 points.
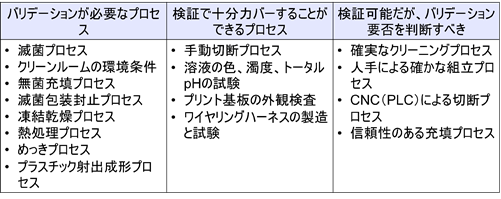
If a 60-point person soldering 100 locations, he or she will screw up 40 locations. This is not the way to manufacture safe medical devices. As an example, the figure below shows a process that requires validation, a process that can be adequately verified by verification, and a process that can be verified but should be determined whether or not validation is required.
related product
[blogcard url=https://xn--2lwu4a.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/O017.html title=”【VOD】ISO 13485における医療機器の洗浄バリデーションの進め方および統計的手法とサンプルサイズ設定根拠セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/QMS-MHLW-28.html title=”【2021年度改正QMS省令対応】プロセスバリデーション規程・手順書・様式”] [blogcard url= https://ecompliance.co.jp/SHOP/MD-QMS-122.html title=”【ISO-13485:2016対応】プロセスバリデーションひな形セット”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-116.html title=”【セミナービデオ】【医療機器】統計的手法によるサンプルサイズ決定方法セミナー”]]]>


Comment