Why Data Integrity is Hard for Companies
Interest in data integrity is growing.
However, all companies are currently struggling to deal with data integrity.
The reasons why data integrity compliance is hard for companies are as follows
- Performance and business pressures
- Lack of awareness or incapacity
- DI is not ingrained in the corporate culture.
- Inappropriate processes and technology
Performance and business pressures
All companies are busy with drug development, drug manufacturing, etc. As a company, the pursuit of sales and profits is unavoidable. However, the priority on business has led to a neglect of data integrity.
Lack of awareness or incapacity
They are unaware of the extent to which their work is related to data integrity. They do not consider the health risks to patients and subjects if data integrity is compromised, nor do they consider the reliability of the application materials.
First, it would be important for all employees to become aware of the importance of data integrity.
However, even if they realize the importance of data integrity, they may not have enough personnel to double-check the data, or they may not have the ability (knowledge and experience) to extract data errors, etc. in the first place.
DIis not ingrained in corporate culture
The goal now should be to foster a “Quality Culture“. All employees, from the board down, must have a culture that values data integrity.
The author has seen many companies’ SOPs through consultations. In such cases, I sometimes ask, “Why is this process done this way? In many cases, the answer is, “I don’t know why, but it seems we have been doing it that way for quite some time. The answer is, “We have been doing it that way for quite some time.
This is not a data integrity response.
What is needed is to spend time and effort to continuously improve SOPs and mitigate any risks. The tendency to think that there is no problem as long as the procedures are implemented according to the previous ones must be changed.
Inappropriate processes and technology
As noted above, all risks must be mitigated in the SOP.
In many companies, is it not a procedure that is supposed to proceed in sequence?
An important part of the data integrity response is to anticipate cases that do not proceed in order.
For example, if there is a process such as “transcribing,” there could be risks such as “transcription errors.
If there is a process such as “calculating,” there is a risk of “calculation error.
If there is a process such as “inputting information,” there could be risks such as “inputting errors.
In order to detect them and prevent problems from occurring, for example, double-checking could be implemented.
The current process, which does not take into account risk, must be reviewed urgently.
In MS-Excel, there are also security risks and audit trail risks.
Furthermore, simple systems such as electronic balances, TOC, and PH meters are not directly connected to the database and involve human-mediated transcription.
This use of inappropriate technology is itself a setback in data integrity measures.
Human error should be addressed.
Here are some interesting data.
The biggest cause of data integrity violations is “inadvertent errors. In other words, human error. Inadvertent errors account for 80% of all data integrity violations.
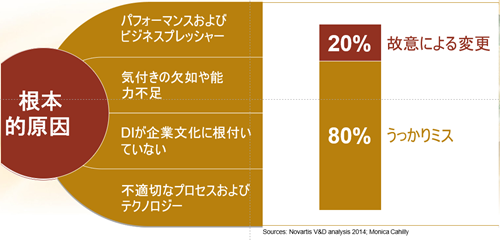
The remaining 20% are “intentional changes. However, “intentional change” does not necessarily mean “malicious intent.
The person in question corrects the data because he or she thinks it is correct, but the correction may be a mistake.
This arises from assumptions, misunderstandings, and lack of education.
When listening to lectures by regulators, they often emphasize the importance of “fraud prevention. Therefore, many companies seem to mistakenly believe that data integrity measures are about fraud prevention.
However, I have to ask. Is “injustice” such an everyday occurrence in the world?
The first thing that should be addressed is human error.
Also, if you listen to many data integrity seminars, etc., they only mention the security of electronic records.
Data integrity is not limited to electronic records. Paper records (handwritten records) should be equally addressed. Which is more serious to the patient, falsification of electronic records or falsification of paper records? Both are the same.
related product
[blogcard url= https://xn--2lwu4a.jp/qms-csv/ title=”QMS(手順書)ひな形 CSV関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/EL-006.html title=”【セミナービデオ】データインテグリティSOP作成セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/O022.html title=”【VOD】医薬品の品質試験における信頼性基準適用の考え方と問題事例セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/P139.html title=”【書籍】 当局要求をふまえた データインテグリティ手順書作成の要点”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-120.html title=”【セミナービデオ】データインテグリティの誤解と要点”]]]>


Comment