Continuous improvement of data integrity and
Article 20.2 of the GMP Ministerial Ordinance is a new article established by the 2021 revision to ensure data integrity in Japan.
Article 20 Management of Documents and Records
2 Manufacturers, etc. shall have a person designated in advance to perform the following tasks with respect to the procedure manuals, etc. and the records prescribed in this chapter, based on the documents prescribed in Article 8, paragraph 2.
(i) To continuously manage the procedure manuals and records to be prepared and kept so that there are no omissions.
(ii) To maintain the prepared procedures, etc. and records to ensure their accuracy on an ongoing basis.
(iii) Continuously manage the contents of other procedures and records to ensure that there are no inconsistencies with the contents of other procedures and records.
(iv) In the event that any omission is found in the procedures, etc. or records, or that any inaccuracy or inconsistency is found in their contents, the cause thereof shall be investigated and the necessary corrective and preventive measures shall be taken.
(v) Other tasks necessary to ensure the reliability of procedures and records.
(vi) Prepare and keep records pertaining to the operations described in the preceding items.
As noted above, in Items 1 through 3, data integrity responses are required to be managed on an ongoing basis.
Awareness makes you aware of many problems.
More police officers means more parking tickets. More police officers will increase speeding. This does not mean that more people are parking violations, nor that more people are speeding. It is an increase in the number of opportunities for detection.
Also, if MRs are educated, the number of adverse event reports increases. This is likewise not due to an increase in adverse events per se, but rather an increase in awareness.
Similarly, with regard to data integrity, it is impossible to know whether there are no data integrity violations because one thinks there are no data integrity violations, or whether there really are no data integrity violations, without a close examination of the data integrity violations.
First, it is necessary to extract various risks that threaten data integrity (transcription errors, input errors, calculation errors, operational errors, etc.) and reconfirm the reliability of data in the past.
The number of data integrity violations detected would increase dramatically if we were aware of the risks in each process. This is a short-term data integrity response.
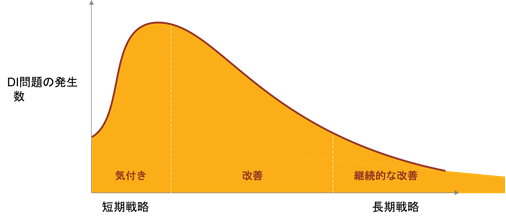
If data integrity violations are detected, CAPAs (corrective and preventive actions) must be implemented for improvement. Improvements will ensure that recurrence and prevention are prevented.
This is a medium-term data integrity response.
Unfortunately, however, repeated improvements will not reduce errors to zero. A small number of data integrity violations will occur. What is important here is to continuously implement data integrity measures.
In other words, data integrity violations must be monitored and remedied on an ongoing basis, much like wringing a dry towel.
This is the continuous management required by the GMP ordinance. There is no end to data integrity measures, and continuous improvement is desirable.
related product
[blogcard url= https://xn--2lwu4a.jp/qms-csv/ title=”QMS(手順書)ひな形 CSV関連” ] [blogcard url= https://ecompliance.co.jp/SHOP/EL-006.html title=”【セミナービデオ】データインテグリティSOP作成セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/O022.html title=”【VOD】医薬品の品質試験における信頼性基準適用の考え方と問題事例セミナー”] [blogcard url= https://ecompliance.co.jp/SHOP/P139.html title=”【書籍】 当局要求をふまえた データインテグリティ手順書作成の要点”] [blogcard url= https://ecompliance.co.jp/SHOP/O100.html title=”【VOD】データインテグリティの誤解と要点”]]]>


Comment