What is Process Validation
In medical device manufacturing, many inspections are nondestructive. Therefore, inspection of all products is possible.
In the unlikely event that nonconforming products are detected, they should be taken off the line and not shipped.
However, some processes may involve destructive testing.
Examples include soldering, crimping, sterilizing, gluing, crimping, and welding.
However, if the product is destroyed, it will not become a product, so sampling inspections are inevitable. In other words, it is difficult to inspect all products.
Such a process in which a full product inspection cannot be performed is called a “special process.
In other words, for special processes, verification (inspecting after manufacturing to assure quality) is not sufficient, and validation (assuring quality to a high degree in advance) is required.
ISO 13485 has the following requirements for process validation
7.5.6 Process validation for manufacturing and service provision
If the outputs resulting from the manufacturing and service provision process cannot or will not be verified by subsequent monitoring or measurement, and as a result, defects will only become apparent after the product has been used or the service has been provided, the organization shall perform the applicable process validation of its manufacturing and service provision. The organization performs the applicable process validation of its manufacturing and service provision.
Validation will demonstrate that these processes consistently deliver the planned results.
Here, “impossible to verify by subsequent monitoring or measurement” is a special process. Also, “or no verification is performed” is a process that is difficult to inspect for reasons of inspection cost, for example. In other words, it is a case where it is cheaper to perform validation than to perform a full product inspection (verification).
It also states that “Validation demonstrates that these processes consistently deliver the planned results.
In the manufacture of medical devices, it must be possible to continuously (consistently) produce products that meet the required specifications and quality.
In other words, it must be possible to manufacture the first lot, the 10th lot, the 100th lot, the 1000th lot, and the 10,000th lot with the same quality and to the same specifications.
In other words, they must be able to manufacture to the same specifications and with the same quality one year, three years, five years, and ten years from now.
Validation is future tense and refers to a high degree of quality assurance that everything will be in accordance with specifications in the ongoing production. It must also be able to explain this high level of quality assurance with documented evidence.
Process validation required.
In the ISO 13485 Practical Guide, there are examples of “processes that require validation,” “processes that can be adequately power-barred by verification,” and “processes that can be verified but should be determined whether or not validation is required.
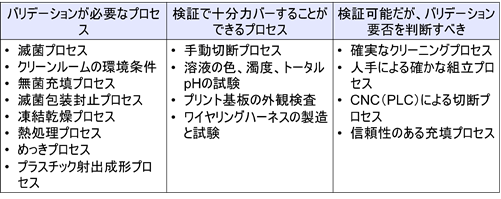
Process validation is mandatory for the following processes because they are special processes.
- Sterilization Process
- Clean room environmental conditions
- Aseptic Filling Process
- Sterile packaging and sealing process
- Freeze-drying process
- Heat Treatment Process
- Plating Process
- Plastic injection molding process
The following do not require validation and can be verified.
- manual cutting process
- Testing of solution color, turbidity, and total pH
- Visual inspection of printed circuit boards
- Wiring harness manufacturing and testing
In addition, the following processes must be determined by the medical device company, depending on the product, whether or not process validation is required.
- Reliable cleaning process
- Reliable assembly process by hand
- Cutting process by CNC (PLC)
- Reliable filling process
In other words, cleaning validation is not necessarily mandatory and must be determined on a case-by-case basis.


Comment