What is GxP data?
The term GxP may be unfamiliar to some readers.
GAMP 5, published in 2008, is titled “A Risk-Based Approach to Compliant GxP Computerized Systems” and uses the term GxP.
So what exactly is the definition of GxP?
Article 1 of the Pharmaceutical Affairs Law (Law Concerning Assurance of Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices and Other Products) states the purpose.
chapter one general rules
(Objective.)
Article 1 This Act provides for the necessary regulations to ensure the quality, efficacy and safety of pharmaceuticals, quasi-drugs, cosmetics, medical devices and regenerative medical products (hereinafter referred to as “pharmaceuticals, etc.”) and to prevent the occurrence and spread of health hazards due to their use. The purpose of this Act is to improve health and sanitation by implementing regulations necessary to ensure the quality, efficacy and safety of pharmaceuticals, quasi-drugs, cosmetics, medical devices and regenerative medical products (hereinafter referred to as “pharmaceuticals, etc.”) and to prevent the occurrence and spread of health hazards due to their use, by taking measures to control designated drugs, and by taking measures necessary to promote research and development of pharmaceuticals, medical devices and regenerative medical products that are particularly necessary from a medical standpoint. The purpose of the Act is to improve health and hygiene by taking necessary measures to prevent the occurrence and expansion of designated drugs.
In other words, what is important in pharmaceuticals, quasi-drugs, cosmetics, medical devices, and regenerative medicine products is to ensure quality, efficacy, and safety.
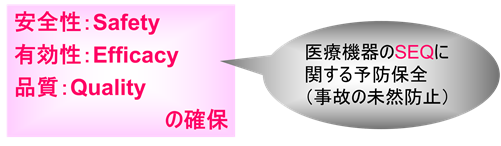
SEQ stands for Safety, Efficacy, and Quality.
The data associated with SEQ is called GxP data.
In other words, GxP data is data that is subject to regulation under the Pharmaceutical Affairs Law.
GxP data includes data directly related to SEQ and data indirectly related to SEQ.
Data directly related to SEQ include manufacturing records, quality test records, validation records, calibration records, case records, adverse event data, and non-clinical study records.
On the other hand, data that indirectly affect SEQ include education records and SOPs. For example, although education records are regulated data, even if education records are falsified or lost, they do not directly affect patient safety or product quality.
Systems that handle GxP data are referred to as GxP systems; GxP systems are subject to CSV. Conversely, non-GxP systems (e.g., meeting room reservation systems) are not regulated, and therefore CSV implementation is not mandatory.



Comment