What is the 3-step method?
There are numerous standards for risk management, both ancient and modern. However, all international standards need to follow ISO/IEC Guide 51 “Safety Aspects – Guidelines for implementation in standards”, jointly developed by ISO and IEC. The same applies to ISO 14971. In Guide 51, the “3-step method” is used. The “3-step method” is a set of safety measures and procedures to be implemented by the designer, consisting of: 1. intrinsic safety design measures, 2. safeguards and additional protection measures, and 3. provision of information about residual risks.
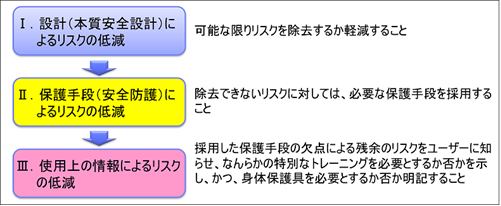
Safety principles (3 step method)
When the PL Law was enacted in Japan in 1995, activities to warn users of the dangers in instruction manuals and caution labels were strengthened. Although it is important to warn users of hazards, there is a limit to the amount of attention that can be paid by humans, and not much can be hoped for in terms of risk reduction. The greater the risk, the less sufficiently risk reduction can be achieved without the use of intrinsic safety design and protective devices.
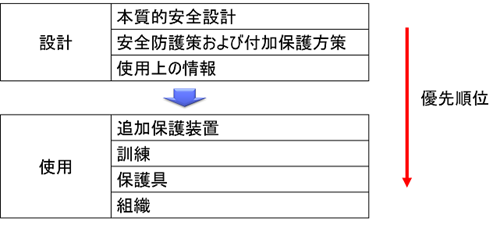
Priority of risk reduction measures
To reduce serious risks, measures on the part of the manufacturer alone are not sufficient. Appropriate risk control measures are also needed on the user side. “1. additional protective equipment”, “2. training”, “3. protective equipment”, and “4. organization”. In risk reduction measures, lower level measures should not be taken while omitting higher level measures. The application of measures shall be based on risk assessment.
related product
[blogcard url=https://xn--2lwu4a.jp/qms-md/ title=”QMS(手順書)ひな形 医療機器関連” ] [blogcard url=https://ecompliance.co.jp/SHOP/EL-008.html title=”【セミナービデオ】【手順書付き】医療機器リスクマネジメントセミナー” ] [blogcard url=https://ecompliance.co.jp/SHOP/O090.html title=”【VOD】【医療機器】リスクマネジメントの具体的な実施方法セミナー(ISO 14971:2019準拠) 【リスクマネジメント手順書配布・実習付き】”] [blogcard url= https://ecompliance.co.jp/SHOP/MD-QMS-026.html title=”【ISO 14971:2019対応】リスクマネジメント規程・手順書・様式”] [blogcard url= https://ecompliance.co.jp/SHOP/EL-110.html title=”【セミナービデオ】医療機器企業における設計管理・リスクマネジメント・バリデーションセミナー ~バリデーション編~”]]]>

Comment